Synthesis of chiral metal-organic frameworks by post‐synthetic modification
27 May 2020
Abstract
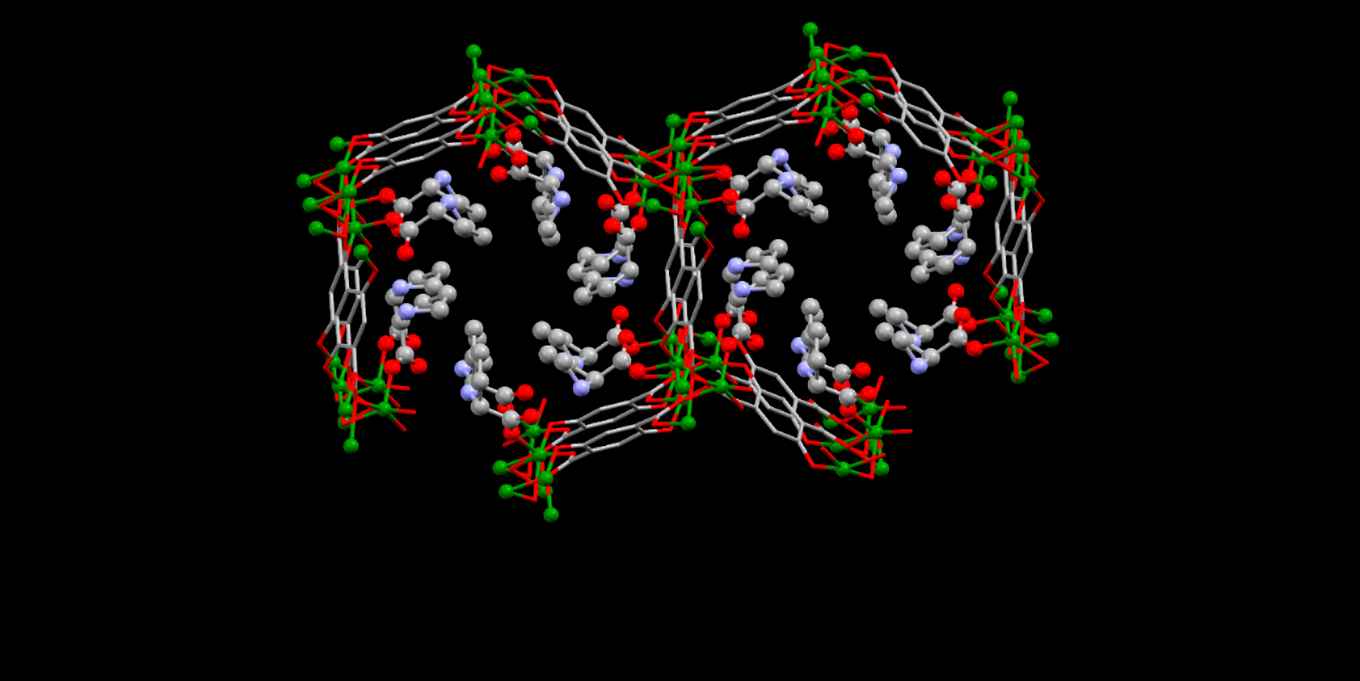
The synthesis of chiral metal‐organic frameworks (MOFs) is highly relevant for asymmetric heterogeneous catalysis, yet very challenging. Chiral MOFs with MOF‐74 topology were synthesized using post‐synthetic modification with proline. Vibrational circular dichroism studies demonstrate that proline is the source of chirality. The solvents used in the synthesis play a key role in tuning the loading of proline and its interaction with the MOF‐74 framework. In N,N’‐dimethylformamide, proline coordinates monodentate to the Zn2+ ions within the MOF‐74 framework, whilst it is only weakly bound to the framework when using methanol as solvent. Introducing chirality within the MOF‐74 framework also leads to the formation of defects, with both the organic linker and metal ions missing from the framework. The formation of defects combined with the coordination of DMF and proline within the framework leads to a pore-blocking effect. This is confirmed by adsorption studies and testing of the chiral MOFs in the asymmetric aldol reaction between acetone and para ‐nitrobenzaldehyde.
Publication
Andreea Gheorghe, Benjamin Strudwick, Sander Woutersen, David Dubbeldam, Stefania Tanase, Synthesis of chiral MOF-74 frameworks using post-synthetic modification by an amino acid. Chemistry - A European Journal, Accepted 27 May 2020, DOI: 10.1002/chem.202002293.