Intricate nanospheres containing hydrogenase mimics for proton reduction catalysis
Communication in Angewandte Chemie
15 July 2020
Abstract:
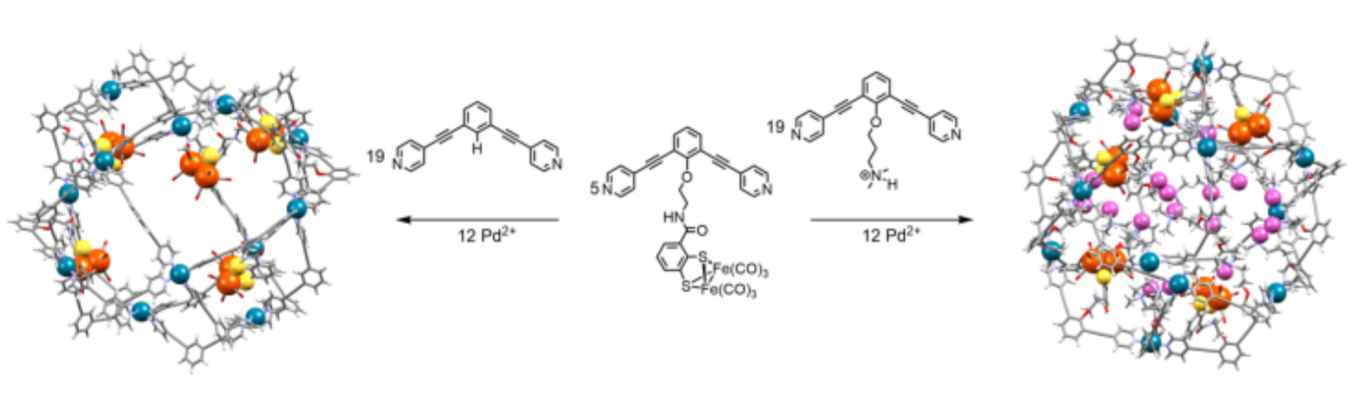
Hydrogenase enzymes are excellent proton reduction catalysts and therefore provide clear blueprints for the development of nature-inspired synthetic analogues. Mimicking their catalytic centre is straightforward but mimicking the protein matrix around the active site and all its functions remains challenging. Synthetic models lack this precisely controlled second coordination sphere that provides substrate preorganization and catalyst stability and, as a result, their performances are far from those of the natural enzyme. In this contribution we report a strategy to easily introduce a specific yet customizable second coordination sphere around synthetic hydrogenase models by encapsulation inside M 12 L 24 cages and at the same time create a proton‐rich nano‐environment by co‐encapsulation of ammonium salts, effectively providing substrate preorganization and intermediates stabilization. We show that catalyst encapsulation in these nano‐cages reduces the catalytic overpotential for proton reduction by 250 mV as compared to the uncaged catalyst while the proton‐rich nano‐environment created around the catalyst ensures that high catalytic rates are maintained.
Paper:
Riccardo Zaffaroni , Nicole Orth, Ivana Ivanović-Burmazović, Joost Reek: Hydrogenase mimics in M12L24 nano‐spheres to control overpotential and activity in proton reduction catalysis Angewandte Chemie International Edition, First published: 02 July 2020. DOI: 10.1002/anie.202008298