Designable catalytic materials combine the best of both worlds
UvA students demonstrate unique concept for making highly stable materials with perfect spatial and chemical control
14 September 2020
One of the biggest challenges of sustainable chemistry is finding materials that are versatile enough to catalyse different reactions, yet stable enough to withstand harsh industrial conditions. Many academic studies report new materials and new catalysts, but few of these discoveries actually reach large-scale application. Those that do are often the product of serendipity – the rare "happy accident" that gives materials that are not only active and selective, but also sufficiently stable to run hundreds and thousands hours on stream. The challenge in designing such materials is that you must be able to set in advance where the active sites will be and what will be their chemical environment. Such designed materials are all too often expensive and fragile.

A team of researchers of the UvA's Research Priority Area Sustainable Chemistry, led by Prof. Gadi Rothenberg, has now succeeded in addressing this challenge using a structured bottom-up approach. Instead of testing different materials, the researchers designed stable catalytic building blocks and connected them with strong linkers. The result is a set of covalently-bonded polymers, with well defined spatial and chemical properties, that can catalyse a variety of reactions.
The materials were made by PhD student Ilse Denekamp and master student Connor Deacon-Price working as part of the TOP-PUNT project Catalysis in Confined Spaces. They mimic the effectiveness of natural systems, where enzymes catalyse reactions that chemists can only dream of with envy. Many of these enzymes rely on single-atom or dual-atom sites. Their superb spatial and chemical control enables them to carry out selective transformations under very mild conditions. Borrowing concepts and pieces of the natural puzzle, the team used these for designing new active materials. Yet unlike nature, which needs millennia to run evolutionary cycles, the UvA researchers used chemistry to “mix & match” these concepts and properties, creating solid materials that can function under harsh industrial conditions: high temperatures, pressures, and corrosive environments.
The best of both worlds
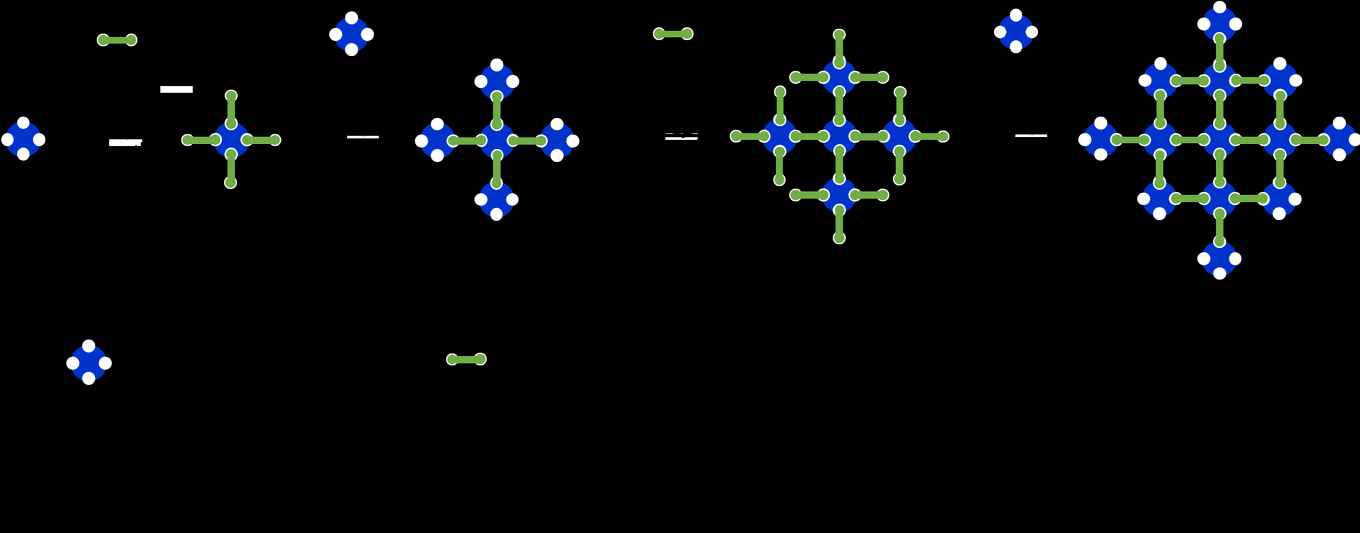
The team focussed on oxygen activation and oxidation reactions, where selectivity and stability are a must. Here, nature typically uses the haem function, comprising a porphyrin macrocycle ligand with nitrogen atoms surrounding a single central metal atom. The active site is extremely well-defined, but the system is delicate, requiring a cumbersome protective protein shell. "What we wanted is the best of both worlds", explains Rothenberg; "We wanted to combine the specificity of the haem complex with the stability and functional flexibility of modern-day industrial materials. Even more importantly, we want a flexible approach, where we can easily change the type of metal site, the distance between sites, and the properties of the surroundings. Last but not least, the new materials had to be scalable and affordable, so that they could be used in real-life applications".The researchers turned to classic organic chemistry, using metallophthalocyanines as their single-atom sites and connecting these with strong amide bonds. Building on a simple and effective synthesis of phthalocyanines developed by Denekamp in 2019, they created a sequential protocol which could give single-atom sites with near-perfect spatial and chemical control. In this process (see figure below) concentric "shells" of active sites are connected in sequence, giving catalytic polymers that remain stable at temperatures as high as 500 ºC. The "connectors" can be changed, creating a myriad of possible materials using a small set of chemical reactions.
Too stable for analysis?
Once the materials were prepared and tested, an unusual problem surfaced: The new polymers were so stable that there was almost no way to determine their structure. They are practically insoluble, regardless of solvent and conditions, precluding traditional polymer analysis methods. They are also amorphous, ruling out X-ray diffraction. Moreover, as carbonaceous molecular species, they are too small and too transparent for microscopy analysis. Three methods, however, were possible: elemental analysis, vibrational spectroscopy (FTIR) and X-ray absorption spectroscopy (XAS). The first two were available at the Amsterdam Science Park, but XAS requires a synchrotron, and getting access to one, especially with the covid-19 pandemic, proved difficult. "The earliest measuring slot that we could find in Europe would have taken at least six months" says Rothenberg. "Instead, we reached out to colleagues in China, Prof. Weixin Huang from USTC and Prof. Zhenhua Zhang from Zhejiang Normal University, who could help us. It took three days to FedEx the samples to China, and a week later we had detailed measurements in our inbox".
According to Rothenberg the results, published as an open-access paper in Catalysis Science & Technology, are just the beginning. "We have now a simple and effective tool for making catalysts by design, where we know for certain where the active atoms are and what is their environment – this adventure is just starting".
Original open-access article:
I.M. Denekamp, C. Deacon-Price, Z. Zhang and G. Rothenberg: Covalent structured catalytic materials containing single-atom metal sites with controllable spatial and chemical properties: concept and application, Catal. Sci. Technol., 2020, published online. DOI: 10.1039/D0CY01299H (Open Access).
Links
Website Heterogeneous Catalysis & Sustainable Chemistry group.