Quantum dots for electrocatalytic oxidation
30 September 2020
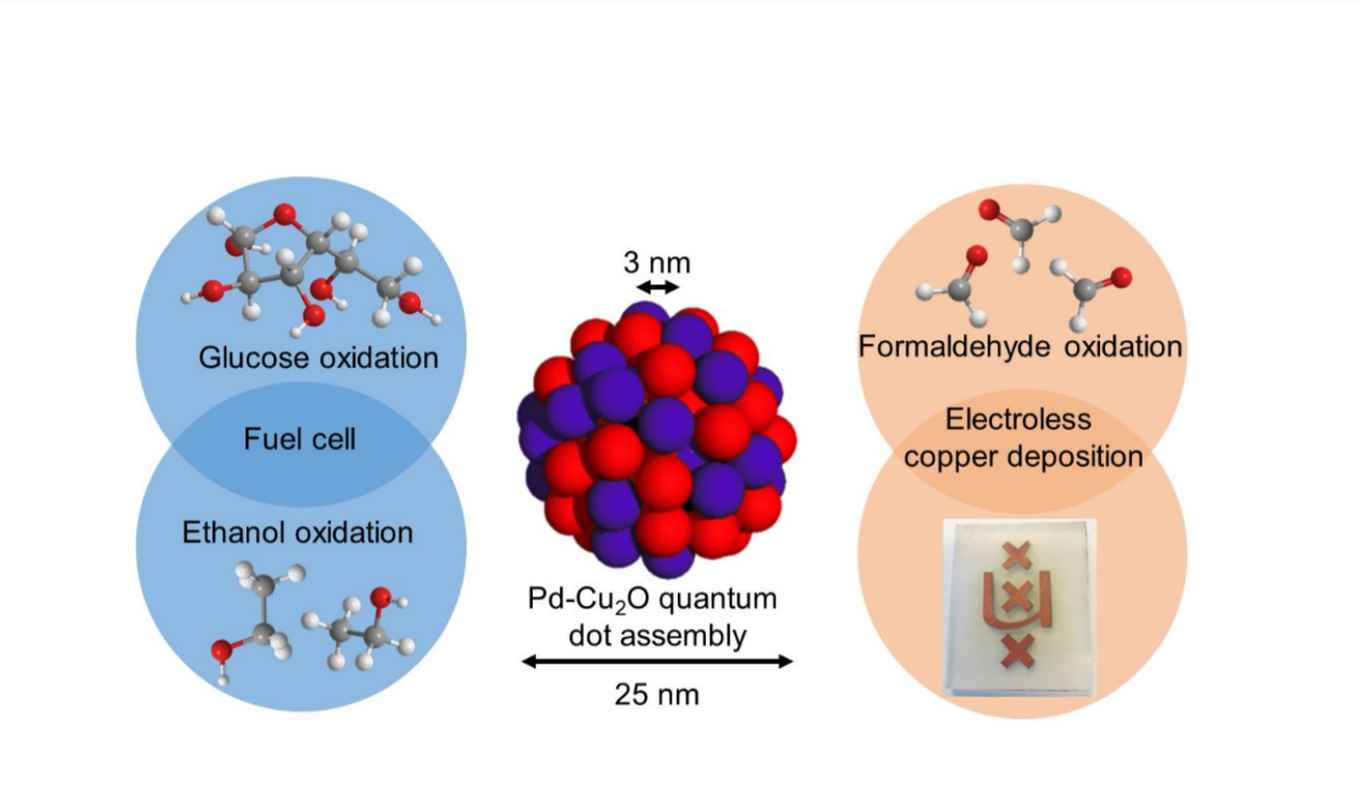
Abstract
To effectively manipulate the electronic structure of the catalysts, we present here a simple bottom-up synthesis protocol for agglomerating palladium and cuprous oxide ultrasmall nanoclusters into single nanoparticles, forming so-called quantum dot assemblies (QDAs). Our synthesis is based on the galvanic displacement of copper with palladium cations at O2-free conditions, rendering the simultaneous and unique crystal growth of ~3 nm Cu2O and Pd clusters. Such assemblies, comprising ultrasmall nano-constitutes, offer much more phase boundaries, where the interfacial electronic effect becomes prominent in catalysis. This is demonstrated in the electrocatalytic oxidation of formaldehyde, ethanol and glucose. In all three cases, the QDA catalyst, despite its lower Pd loading, outperforms the monometallic palladium catalyst. Indeed, complementing the experimental results with density-functional theory calculations, we could confirm the sharply increased charge density at the Pd-Cu heterojunction and the decreased energy barrier of the formaldehyde oxidation on the QDA catalyst. Finally, we applied these catalysts in electroless copper deposition – an industrially relevant process for the manufacturing of printed circuit boards. The QDA catalysts gave uniform and robust copper wires at a rate that was three times faster than the monometallic Pd catalyst, showing their potential for real-life applications.
Paper
Jasper Biemolt, Dylan van Noordenne, Jian-Wen Liu, Elise Antonetti, Manon Leconte, Stefan van Vliet, Roland Bliem, Gadi Rothenberg, Xian-Zhu Fu and Ning Yan: Assembling Palladium and Cuprous Oxide Nanoclusters into Single Quantum Dots for Electrocatalytic Oxidation of Formaldehyde, Ethanol, and Glucose. ACS Appl. Nano Mater. 2020, Published 27 September 2020. DOI: 10.1021/acsanm.0c02162