Improving hydrogen storage with MXene materials
27 November 2020
Using a novel two-dimensional material as support, Thierry Slot, a joint PhD student of both groups, succeeded in changing the charge of catalytic particles, offering a new tool for the catalyst-engineering tool belt. Their results, published as an open-access paper in the international journal 2D Materials, have important implications for catalysts design and sustainable energy applications. Within just two weeks of publication, this paper became one of the ten most read papers of the journal!
Hydrogen generation
Hydrogen is the front-runner in our set of renewable fuels, but its high specific volume requires storage and transportation under high pressure. This is both hazardous and costly. An alternative is binding it using carrier molecules such as ammonia borane. These are safe to transport and can release hydrogen when required, using a catalyst.
Now, an international team lead by UvA scientists Dr Raveendran Shiju (CatEng) and Prof. Gadi Rothenberg (HCSC) has improved such catalysts by using a novel material, MXene, as support. The material has both ceramic and metallic properties, which enable an electronic interaction between MXene and platinum nanoparticles.
Thin oxide layer
The degree of surface oxidation of the MXene support is the key to tuning the charge of the particles. Traditional bulk oxide supports have fixed crystal structures and their specific surface modification is difficult. Yet as PhD student Slot explains: “Our method creates a unique thin oxide layer, different from those found on MXene or bulk metal oxides. This layer makes the platinum particles seven times more reactive.”
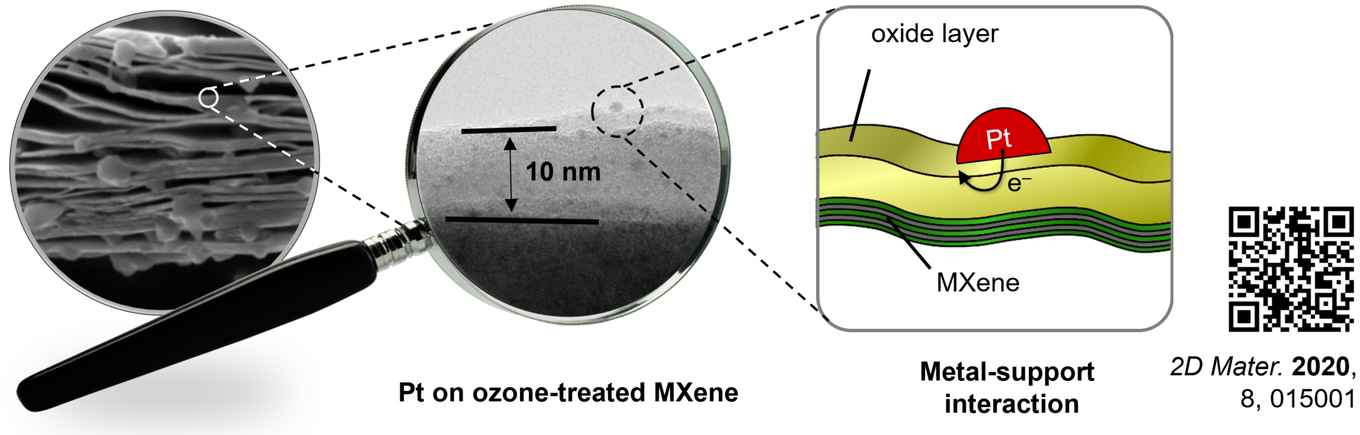
The thin oxide layer on the catalyst surface holds the key to the high reactivity because it withdraws charge from the platinum particles, facilitating binding and reaction of electron-rich ammonia borane at the active sites. Different oxide layers give different metal-support interactions, and thus different catalyst activities. The variety in oxide layers enables the tuning of the charge at the particles, and with it their catalytic performance.
A new family of catalysts
Considering the growing number of MXene compositions and different oxidation methods, there is great potential to apply this technique to other MXene materials. The observed metal-support interaction offers a general tool for tuning the charge of particles and controlling their reactivity, not just for hydrogen generation reactions. Combining this with the increasing number of known MXene compositions opens exciting opportunities for catalysing chemical reactions.
The research is part of the NWO TOP-PUNT program Catalysis in Confined Spaces, carried out at the UvA Research Priority Area Sustainable Chemistry.
Original open-access paper
T.K. Slot, F. Yue, H. Xu, E.V. Ramos-Fernandez, A. Sepúlveda-Escribano, Z. Sofer, G. Rothenberg, N.R. Shiju: Surface oxidation of Ti3C2Tx enhances the catalytic activity of supported platinum nanoparticles in ammonia borane hydrolysis. 2D Mater. 2020, 8, 015001. DOI: 10.1088/2053-1583/ababef