UvA and AMOLF researchers work together towards CO2 valorisation
First catalytic application of nickel-based coral-like architectures
4 December 2020
CO2 capture and utilisation is a priority research area due to the link of this greenhouse gas to climate change. Several industrial processes emit CO2 to the atmosphere; however, instead of treating it as a waste and a contributor to climate change, CO2 can be a valuable C1 feedstock to make chemicals and fuels.
A research goal of the Catalysis Engineering group (HIMS) is to convert CO2 into useful chemicals. One bottleneck on the efficient conversion of carbon dioxide is that it is highly stable. This translates into high reaction temperatures (>600 °C), which limits the industrial application of thermocatalytic CO2 conversion reactions. Researchers in the group actively work towards finding active, selective and stable catalysts that bring down the application costs of CO2 conversion.
Collaborative study
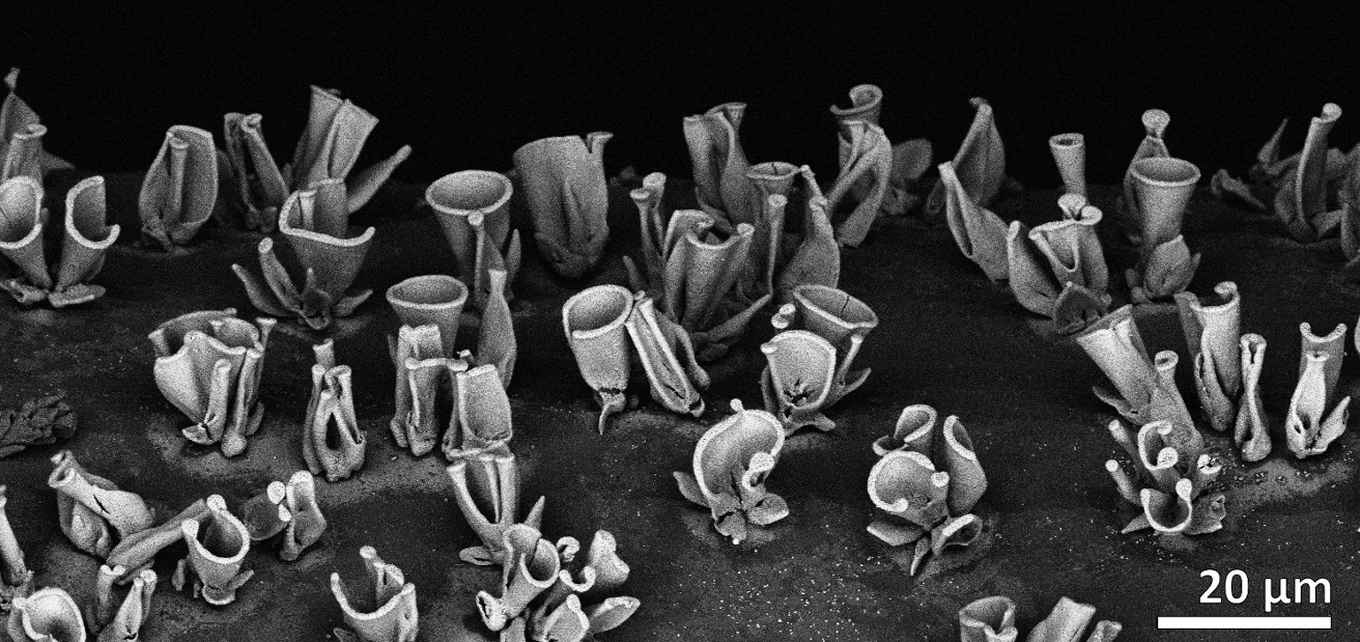
Researchers of the Self-Organizing Matter group (AMOLF) work on making complex and functional, yet beautifully shaped nanocomposites. They recently found that ion exchange can change the composition of these nanocomposites, allowing a large variety of compositions that inherit their sculpted architecture. Specifically, the nanocomposite’s composition can be tuned to nickel-, iron-, manganese- or cadmium salts, making them attractive for catalysis.
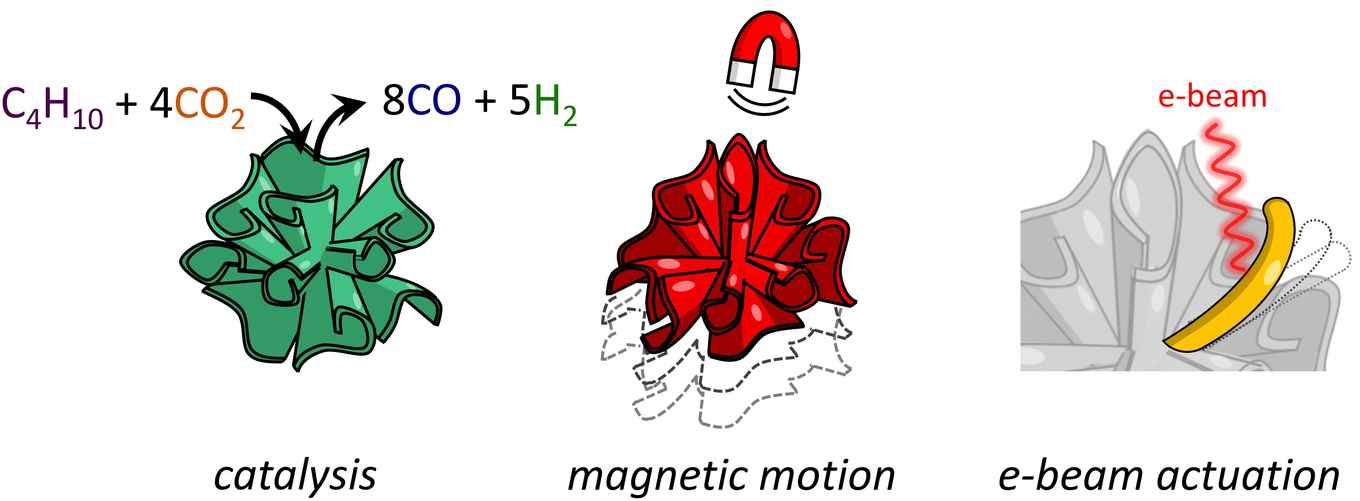
A collaborative study conducted by PhD students Hans Hendrikse (AMOLF) and Maria Ronda-Lloret (HIMS), under the supervision of Dr Wim Noorduin and Dr Raveendran Shiju, recently showed that these coral-like architectures are efficient catalysts for CO2 conversion. They tested a NiO/SiO2 composite in the dry reforming of butane, where butane and CO2 react to form syngas (a mixture of CO and H2). Syngas is a useful chemical mixture to produce a large variety of chemicals, such as hydrocarbons or alcohols through the Fischer-Tropsch synthesis.
Coral-like structures
The coral-like structures allow high metal contents while maintaining a small nanocrystal size and a good contact between their components. This enhanced their interaction with the reagents, achieving high conversion and selectivity at relatively low temperature (400 °C). These coral-shaped catalysts outperform traditional catalysts at low temperatures: a conventional NiO supported on SiO2 catalyst of similar metal content showed very low selectivity to syngas, while a conventional catalyst with lower metal content was inactive at 400 °C.
The team also explored other applications of these new materials. For example, they synthesised shape-controlled magnetite (Fe3O4) nanocomposites that can be moved and reoriented using their magnetic properties. They also created electron-beam activated microscopic actuators by using the flexibility and the shrinking properties of the silica matrix.
This research, supported by the Vernieuwingsimpuls Vidi research program (Shaping up Materials) and a NWO-NSFC grant, is now available as a communication in the international journal Advanced Materials
Publication details
Hans C. Hendrikse, Arno van der Weijden, Maria Ronda-Lloret, Ting Yang, Roland Bliem, N. Raveendran Shiju, Martin van Hecke, Ling Li, and Willem L. Noorduin: Shape-Preserving Chemical Conversion of Architected Nanocomposites, Adv. Mater., 2020, 2003999. DOI: 10.1002/adma.202003999
Read more
- Highlight in Nature: Presto chango: tiny particles get a chemical makeover but keep their shape
- Nature review materials: Nanocomposites keep in shape