Synergetic enhancement between sorption and diffusion in zeolites with continuum intersecting channels
12 March 2021
Abstract
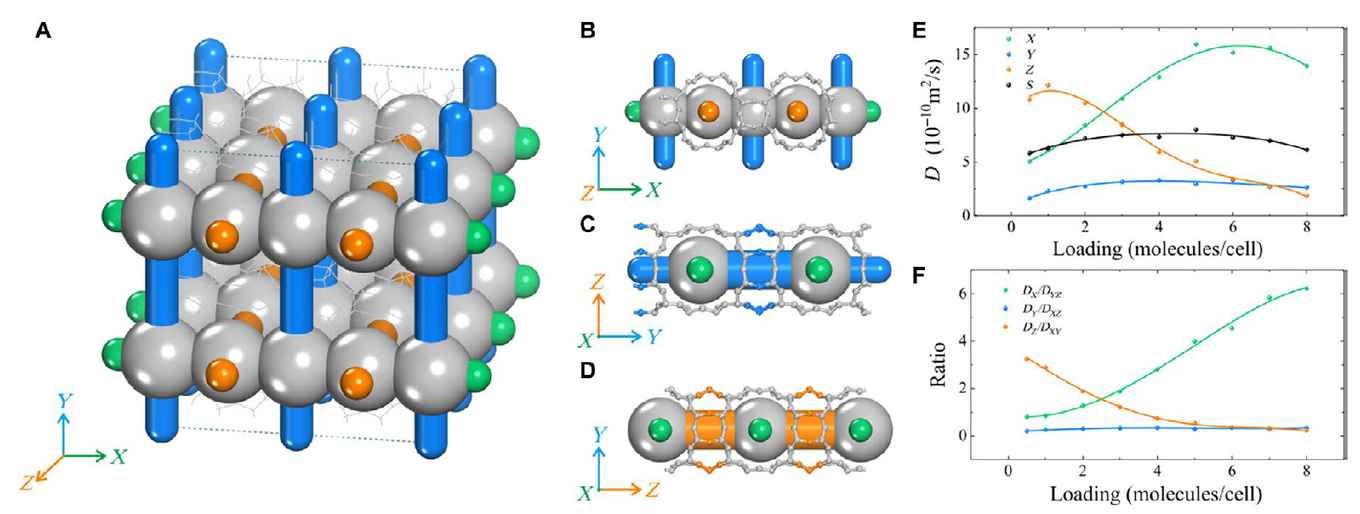
In separation and catalysis applications, adsorption and diffusion are normally considered mutually exclusive. That is, rapid diffusion is generally accompanied by weak adsorption and vice versa. In this work, we analyze the anomalous loading-dependent mechanism of p-xylene diffusion in a newly developed zeolite called SCM-15. The obtained results demonstrate that the unique system of “continuum intersecting channels” (i.e., channels made of fused cavities) plays a key role in the diffusion process for the molecule-selective pathways. At low pressure, the presence of strong adsorption sites and intersections that provide space for molecule rotation facilitates the diffusion of p-xylene along the Z direction. Upon increasing the molecular uptake, the adsorbates move faster along the X direction because of the effect of continuum intersections in reducing the diffusion barriers and thus maintaining the large diffusion coefficient of the diffusing compound. This novel mechanism synergistically improves the diffusion of hydrocarbons in zeolites.
Paper
Zhiqiang Liu, Jiamin Yuan, Jasper M. van Baten, Jian Zhou, Xiaomin Tang, Chao Zhao, Wei Chen, Xianfeng Yi, Rajamani Krishna, German Sastre, Anmin Zheng: Synergistically enhance confined diffusion by continuum intersecting channels in zeolites Sci. Adv. 2021; 7, eabf0775 (2021). DOI: 10.1126/sciadv.abf0775