Electro-oxidation of glycerol for energy conversion and chemical production
9 May 2023
The paper was co-authored by postdoc researcher Cássia Sidney Santana and Amanda Garcia, Principal Investigator Electrochemistry and Electrocatalytic Synthesis at the research group Heterogeneous Catalysis and Sustainable Chemistry. It results from a cooperation with researchers Michael Braun and Corina Andronescu at the University of Duisburg-Essen and is part of the special issue of the journal on Recycling and Reuse within a Circular Economy.
Abstract
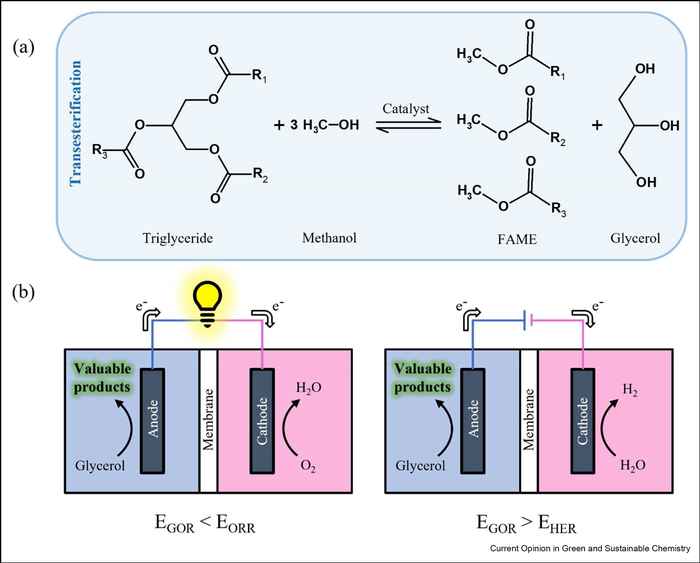
Defossilation of chemical industry, aiming at reducing CO2 emissions, is constrained by the fact that many chemical products used today are carbon-based. To overcome this vicious circle, technologies such as glycerol electrooxidation (GOR) can be used to convert glycerol, a waste product from biodiesel production, into value-added products. In a direct glycerol fuel cell, the value-added products together with electrical energy can be generated, while in an electrolyser, the synthesis of chemicals needs to be powered by renewable energy. However, when scaling up such systems from the laboratory scale to the industrial scale, it is also essential to consider aspects such as market parameters of the potential chemical products and selectivities of the electrocatalysts. In this contribution, we aim to highlight the research opportunities regarding GOR in the context of the transition from fossil to renewable energy resources, with a particular focus on synthesizing GOR products in industrially relevant amounts.
Paper details
Michael Braun, Cássia S. Santana, Amanda C. Garcia, Corina Andronescu: From waste to value – glycerol electrooxidation for energy conversion and chemical production. Current Opinion in Green and Sustainable Chemistry, 2023, 100829 DOI: 10.1016/j.cogsc.2023.100829.