Electrochemical CO2 reduction in an organic solvent using nanostructured copper electrodes
21 July 2023
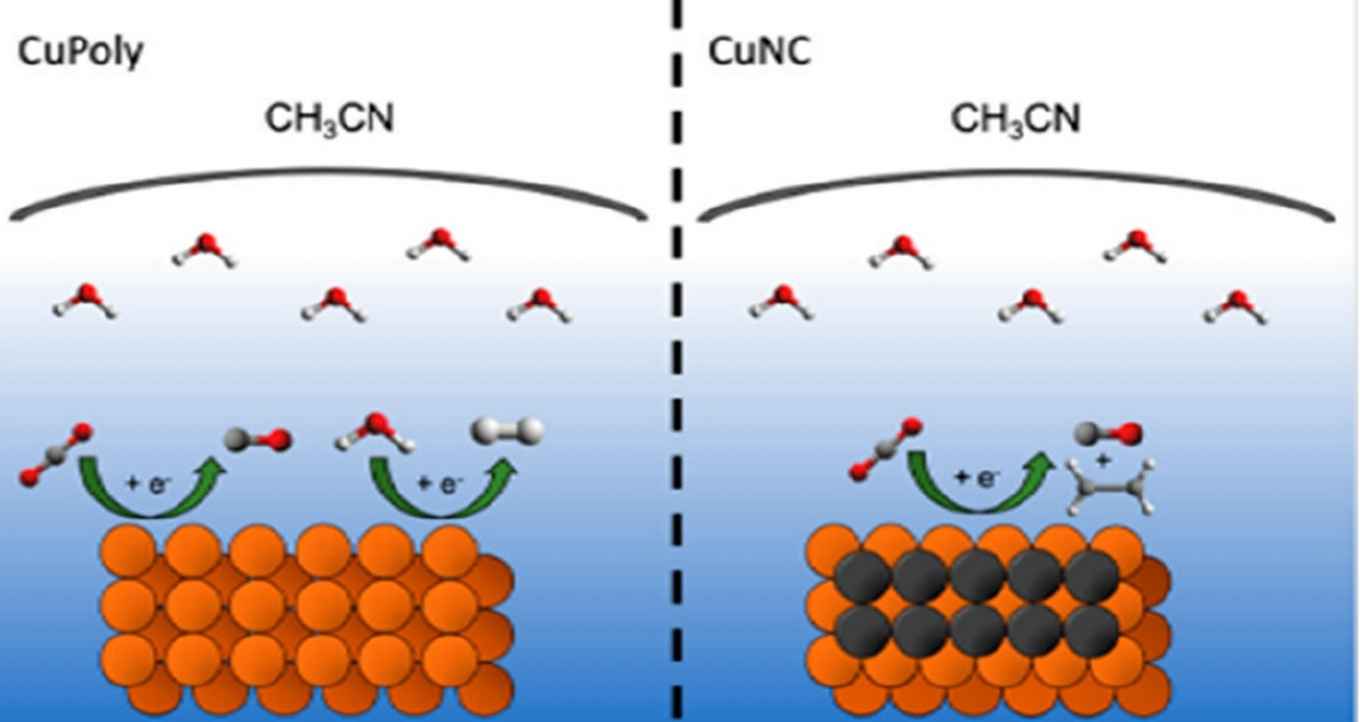
As the chemical industry withdraws from the use of fossil resources, new routes have to be established towards fuels and chemical building blocks. Among these routes is the electrochemical reduction of CO2 using sustainable electricity. A key challenge here is to produce multicarbon (C2+) products that have a high energy density and that can be used as a chemical feedstock in further reactions.
In aqueous conditions it has already been established that the use of copper nanocubes promotes the formation of such C2+ products. However, the use of aqueous electrolytes results in a concomitant hydrogen evolution reaction, reducing the overall system efficiency. This can be avoided by using organic solvents such as acetonitrile. In their J.Phys.Chem.C. paper, PhD student Connor Deacon-Price, postdoc researcher Cássia Sidney Santana and assistant professor Amanda Garcia at the Heterogeneous Catalysis and Sustainable Chemistry group report that on nanostructured copper electrodes, acetonitrile mostly favours CO production. However, when ≥500 mM H2O is present, small amounts C2+ products were also produced, but only on copper nanocubes.
The research was carried out in the framework of the Dutch national programme for Electrochemical Conversion and Materials (ECCM), in cooperation with postdoc researcher Alisson Marques da Silva and professor Marc Koper at the Leiden Institute of Chemistry.
Abstract of the paper
The electrochemical reduction of CO2 (CO2RR) is a sustainable alternative for producing fuels and chemicals, although the production of highly desired hydrocarbons is still a challenge due to the higher overpotential requirement in combination with the competitive hydrogen evolution reaction (HER). Tailoring the electrolyte composition is a possible strategy to favor the CO2RR over the HER. In this work we studied the solvent effect on the CO2RR on a nanostructured Cu electrode in acetonitrile solvent with different amounts of water. Similar to what has been observed for aqueous media, our online gas chromatography results showed that CO2RR in acetonitrile solvent is also structure-dependent, since nanocube-covered copper (CuNC) was the only surface (in comparison to polycrystalline Cu) capable of producing a detectable amount of ethylene (10% FE), provided there is enough water present in the electrolyte (>500 mM). In situ Fourier Transform Infrared (FTIR) spectroscopy showed that in acetonitrile solvent the presence of CO2 strongly inhibits HER by driving away water from the interface. CO is by far the main product of CO2RR in acetonitrile (>85% Faradaic efficiency), but adsorbed CO is not detected. This suggests that in acetonitrile media CO adsorption is inhibited compared to aqueous media. Remarkably, the addition of water to acetonitrile has little quantitative and almost no qualitative effect on the activity and selectivity of the CO2RR. This indicates that water is not strongly involved in the rate-determining step of the CO2RR in acetonitrile. Only at the highest water concentrations and at the CuNC surface, the CO coverage becomes high enough that a small amount of C2+ product is formed.
Paper details
Connor Deacon-Price, Alisson H. M. da Silva, Cássia S. Santana, Marc T. M. Koper, and Amanda C. Garcia: Solvent Effect on Electrochemical CO2 Reduction Reaction on Nanostructured Copper Electrodes. Journal of Physical Chemistry C, 2023, DOI: 10.1021/acs.jpcc.3c03257