Understanding and controlling the reactivity of nitrene radical complexes in water
31 July 2023
(Radical-type) nitrene transfer catalysis is an elegant strategy allowing fast and direct functionalization of C–H and C=C double bonds under mild conditions. The respective reaction products, aziridines for instance, are generally highly valued because they are frequently encountered motifs in pharmaceuticals. However, the synthesis of such motifs typically requires harsh conditions and multiple steps, highlighting the importance of nitrene transfer reactions.
Despite considerable efforts to develop base-metal complexes that facilitate (radical-type) nitrene transfer in organic media, their application in water remains a long-standing challenge. Enabling such transformations in water has the potential to open up a wider range of applications, such as the in vivo synthesis of medicines and other bioactive compounds. Therefore, understanding and controlling the reactivity of nitrene radical complexes in water is of substantial interest.
Summary of the paper
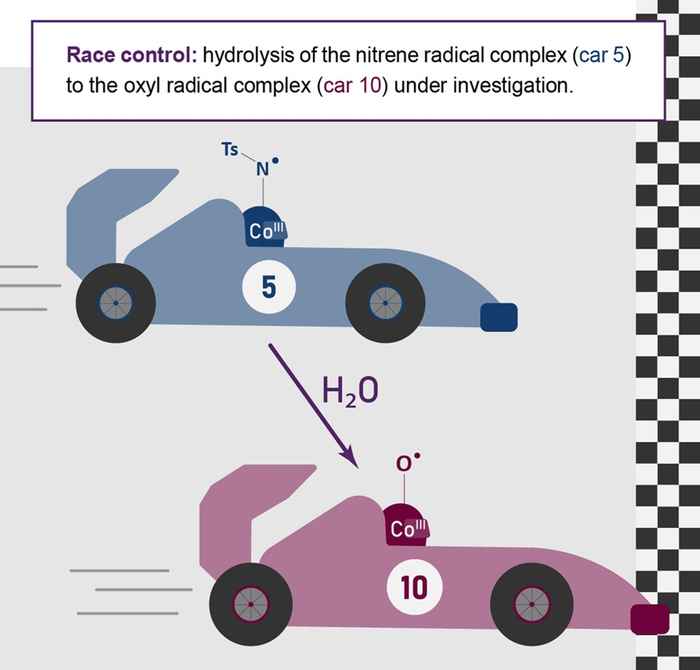
Enabling (radical-type) nitrene transfer reactions in water can open up a wide range of (novel) applications, such as the in vivo synthesis of medicines. However, these reactions typically suffer from oxygen-containing side-product formation, the origin of which is not fully understood. Therefore, Meeus and co-workers investigated aqueous styrene aziridination by using a water-soluble [CoIII(TAMLred)]– catalyst known to be active in radical-type nitrene transfer in organic solvents. The cobalt-catalyzed aziridination of styrene in water (pH = 7) yielded styrene oxide as the major product, next to minor amounts of aziridine product. On the basis of 18O-labeling studies, catalysis, and mass spectrometry experiments (in collaboration with the Roithová group from the Radboud University), the authors demonstrated that styrene oxide formation proceeds via hydrolysis of the formed nitrene radical complexes. Computational studies support that this process is facile and yields oxyl radical complexes active in oxygen-atom transfer to styrene. On the basis of these mechanistic insights, the pH was adjusted to afford selective aziridination in water.
Paper details
Eva J. Meeus, Max T.G.M. Derks, Nicolaas P. van Leest, Caroline J. Verhoef, Jana Roithová, Joost N.H. Reek, Bas de Bruin: Styrene aziridination with [CoIII(TAMLred)]– in water: Understanding and preventing epoxidation via nitrene hydrolysis. Chem Catalysis 2023, 3, 100700. DOI: 10.1016/j.checat.2023.100700
See also
Research group Homogeneous, Supramolecular, and Bio-inspired Catalysis