Heterogenization of a molecular catalyst on a carbon electrode yields surprising results
29 August 2023
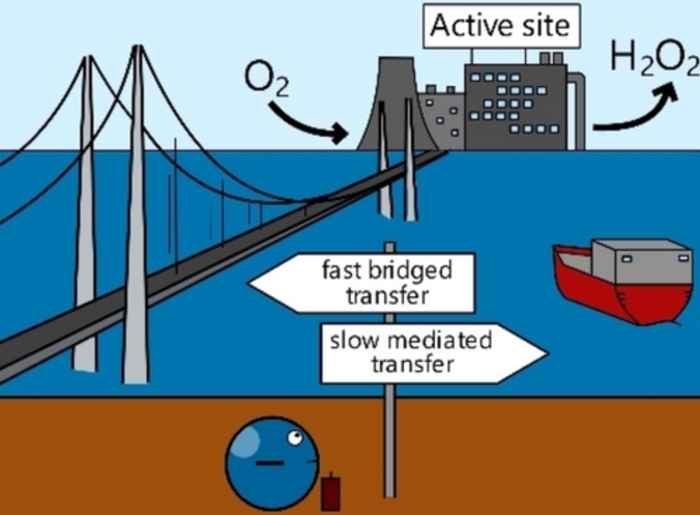
The study was initiated by Biemolt in the last months of his PhD research. It stemmed from an idea he had developed already during his master’s project, wondering if it would be possible to combine well-defined molecular homogeneous catalysts with heterogeneous materials. What started as a simple exploration of grafting a molecular catalyst on an electrode quickly yielded results with potential implications in the field of electrochemistry. For instance, similarities in the electrochemical response were found between the developed catalyst and state-of-the-art graphene conjugated catalysts. Furthermore, the new material efficiently electrochemically reduced oxygen to hydrogen peroxide with nearly no overpotential and over 80% selectivity. Biemolt is currently exploring the concept further as a postdoctoral researcher at Delft University of Technology.
Abstract of the paper
Immobilizing molecular catalysts on electrodes is vital for electrochemical applications. However, creating robust electrode-catalyst interactions while maintaining good catalytic performance and rapid electron transfer is challenging. Here, without introducing any foreign elements, we show a bottom-up synthetic approach of constructing the conjugated C−C bond between the commercial Vulcan carbon electrode and an organometallic catalyst. Characterization results from FTIR, XPS, aberration-corrected TEM and EPR confirmed the successful and uniform heterogenization of the complex. The synthesized Vulcan-LN4−Co catalyst is highly active and selective in the oxygen reduction reaction in neutral media, showing an 80 % hydrogen peroxide selectivity and a 0.72 V (vs. RHE) onset potential which significantly outperformed the homogenous counterpart. Based on single-crystal XRD and NMR data, we built a model for density functional theory calculations which showed a nearly optimal binding energy for the *OOH intermediate. Our results show that the direct conjugated C−C bonding is an effective approach for heterogenizing molecular catalysts on carbon, opening new opportunities for employing molecular catalysts in electrochemical applications.
Details of the paper
Jasper Biemolt, Eva J. Meeus, Felix J. de Zwart, Jeen de Graaf, Petrus C. M. Laan, Bas de Bruin, Thomas Burdyny, Gadi Rothenberg, Ning Yan: Creating Conjugated C−C Bonds between Commercial Carbon Electrode and Molecular Catalyst for Oxygen Reduction to Hydrogen Peroxide ChemSusChem2023,e20230084 DOI: 10.1002/cssc.202300841