Homolytic C–H bond activation by phosphine–quinone-based radical ion pairs
16 October 2023
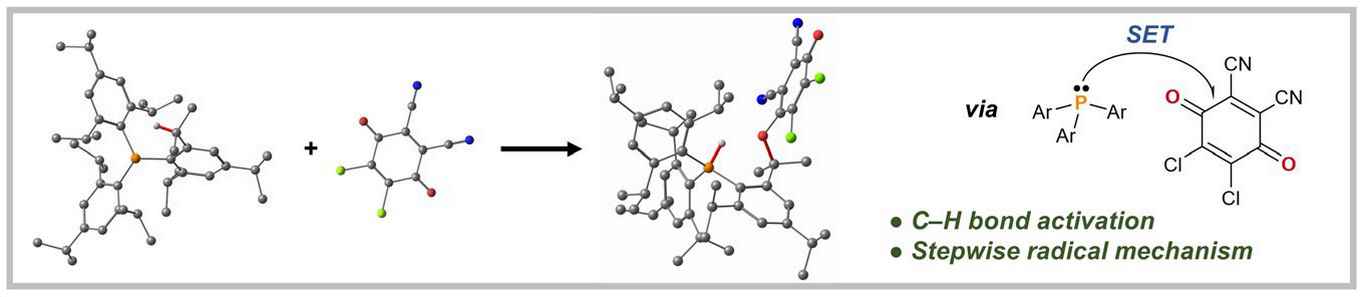
In their paper, Slootweg and co-workers describe the single-electron transfer processes between sterically demanding triarylphosphines and quinones of appropriate electron-accepting character. They report how they were able to obtain either P–O coupled adducts or C–H bond activation products, depending on the steric demand of the phosphine. Experimental and theoretical studies showed that the C–H bond activation proceeds stepwise via transient radical ion pairs. Currently the researchers are exploring the steric and electronic tuning of electron donor–acceptor complexes to enable the C–H bond activation of external substrates by in situ generated radical ion pairs.
Abstract of the paper
Herein, we present the formation of transient radical ion pairs (RIPs) by single-electron transfer (SET) in phosphine–quinone systems and explore their potential for the activation of C–H bonds. PMes3 (Mes = 2,4,6-Me3C6H2) reacts with DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) with formation of the P–O bonded zwitterionic adduct Mes3P–DDQ (1), while the reaction with the sterically more crowded PTip3 (Tip = 2,4,6-iPr3C6H2) afforded C–H bond activation product Tip2P(H)(2-[CMe2(DDQ)]-4,6-iPr2-C6H2) (2). UV-Vis and EPR spectroscopic studies showed that the latter reaction proceeds via initial SET, forming RIP [PTip3]•+[DDQ]•–, and subsequent homolytic C–H bond activation, which was supported by DFT calculations. The isolation of analogous products, Tip2P(H)(2-[CMe2{TCQ–B(C6F5)3}]-4,6-iPr2-C6H2) (4, TCQ = tetrachloro-1,4-benzoquinone) and Tip2P(H)(2-[CMe2{oQtBu–B(C6F5)3}]-4,6-iPr2-C6H2) (8, oQtBu = 3,5-di-tert-butyl-1,2-benzoquinone), from reactions of PTip3 with Lewis-acid activated quinones, TCQ–B(C6F5)3 and oQtBu–B(C6F5)3, respectively, further supports the proposed radical mechanism. As such, this study presents key mechanistic insights into the homolytic C–H bond activation by the synergistic action of radical ion pairs.
Paper details
Christoph Helling, Lars J. C. van der Zee, Jelle Hofman, Felix J. de Zwart, Simon Mathew, Martin Nieger, Chris Slootweg: Homolytic C–H Bond Activation by Phosphine–Quinone-Based Radical Ion Pairs. Angewandte Chemie International Edition, First published: 13 October 2023. DOI: 10.1002/anie.202313397