Iron impurities affect electrochemical oxidation of glycerol on nickel-based electrodes
30 November 2023
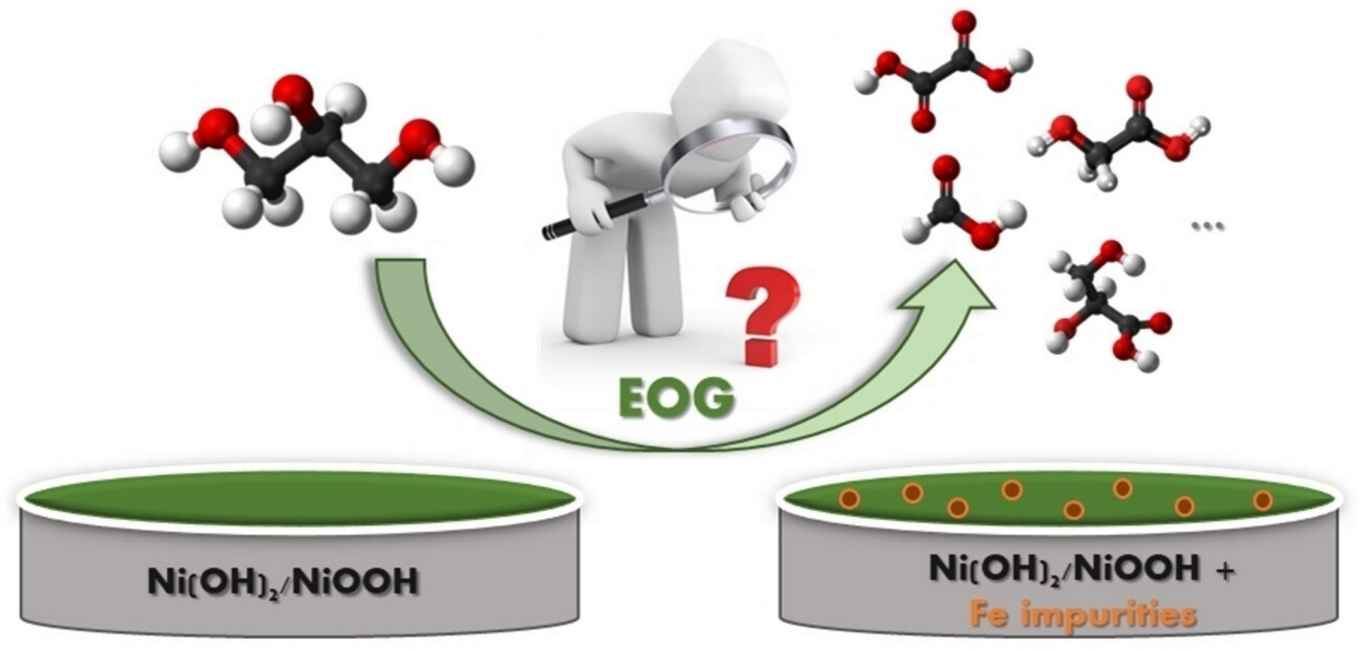
In their paper, the researchers conclude that the incorporation of iron ions and their interaction with the electrode NiOOH structure significantly impact the electrocatalytic activity of glycerol electrooxidation. While Ni functions as the central catalyst, Fe plays a crucial role in stabilizing the reactive Ni−O species with high valence, creating an advantageous electronic environment that, coupled with the alkaline environment, facilitates the C−C cleavage, yielding higher faradaic efficiencies of C2 and C1 products.
Based upon thorough product analyses, the researchers reveal a noteworthy phenomenon: both glyceric (C3) and glycolic (C2) acids undergo oxidation towards formic acid (C1), elucidating the high faradaic efficiency of formic acid compared to glycolic acid on Ni-based electrodes. This effect was particularly pronounced in the non-purified electrolyte, underscoring the intricate interplay between the alkaline environment and iron ions.
Abstract of the paper
Herein, the effect of iron (Fe) ions impurities in alkaline electrolyte is investigated for enhancing the activity of nickel-oxyhydroxide (NiOOH) for electrochemical oxidation of glycerol (EOG). Cyclic voltammetry and chronoamperometry analyses show that Fe impurities have a significant effect on both EOG activity and product distribution. We found the presence of Fe impurities in the electrolyte enhance the C−C cleavage bonds within glycerol and its intermediates, thereby favoring the formation of C2 and C1 products. Our results deviate from the conventional observations in Ni-based catalysis for EOG, which instead of the anticipated glycolate and formate products resulting from the C−C scission of glycerate, we observe the generation of oxalate (C2) and formate (C1), therefore suggesting the involvement of aldehyde intermediates in the reaction mechanism, providing a novel perspective on the higher faradaic efficiency of formic acid compared to glycolic acid. These results show the importance of eliminating iron ions from the electrolyte solution, as this step is pivotal in comprehending the cation effect on the electrochemical oxidation of glycerol in alkaline environments.
Paper details
Cássia S. Santana, Elvisona Gjonaj, Amanda C. Garcia: Effect of Iron Impurities on the Electrochemical Oxidation of Glycerol on Ni(OH)2/NiOOH Electrodes. ChemElectroChem, Early View e202300570, first published 27 November 2023. DOI: 10.1002/celc.202300570
See also
Research group Heterogeneous Catalysis and Sustainable Chemistry