Activation and use of volatile alkanes through photocatalytic hydrogen atom transfer in flow
25 June 2024
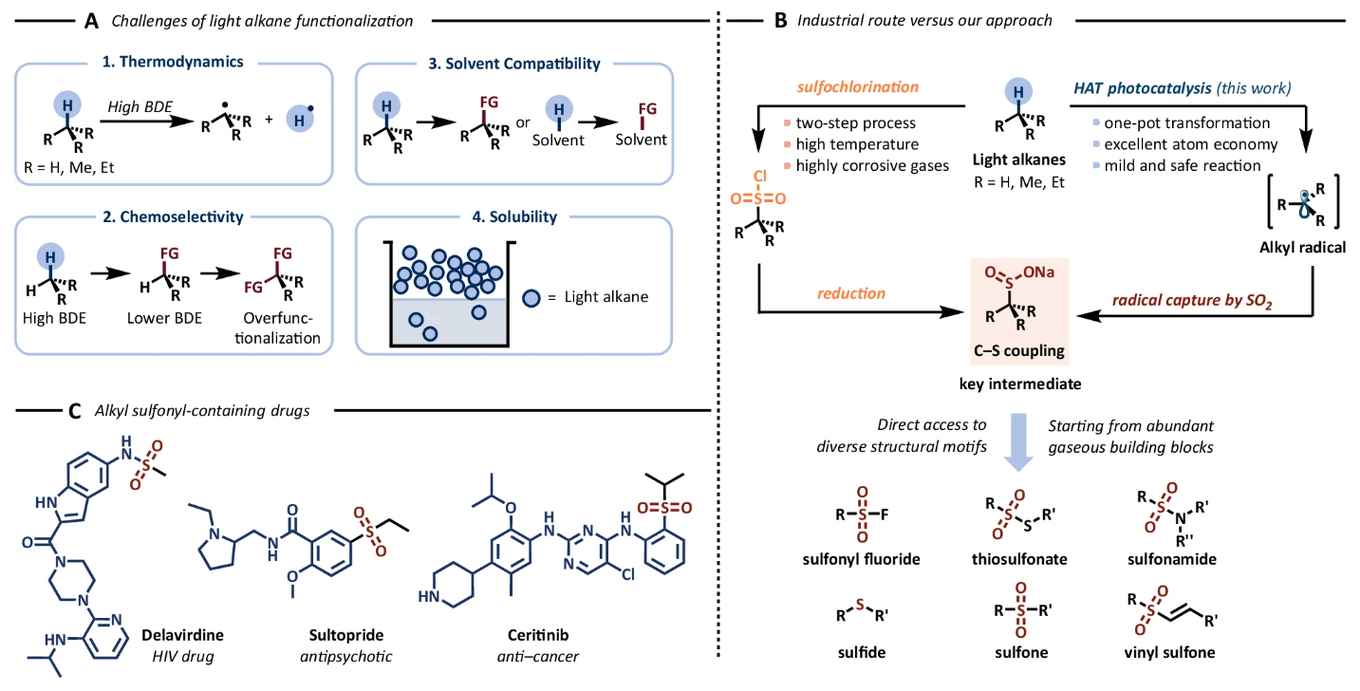
Affordable and atom-economical, gaseous reagents are frequently disregarded due to a combination of chemical and practical considerations. In contrast, this study showcases their facile applicability as versatile reagents within the realm of organic synthesis. The use of flow technology provides a robust, safe, and scalable platform for the HAT-activation of the volatile alkanes. Upon activation they form nucleophilic radicals that react with SO2 to yield corresponding sulfinates. These sulfinates are versatile building blocks, opening new pathways for the synthesis of various sulphur-containing organic compounds such as sulfones, sulfonamides, and sulfonate esters.
Paper abstract
Sulphur-containing scaffolds originating from small alkyl fragments play a crucial role in various pharmaceuticals, agrochemicals, and materials. Nonetheless, their synthesis using conventional methods presents significant challenges. In this study, we introduce a practical and efficient approach that harnesses hydrogen atom transfer photocatalysis to activate volatile alkanes, such as isobutane, butane, propane, ethane, and methane. Subsequently, these nucleophilic radicals react with SO2 to yield the corresponding sulfinates. These sulfinates then serve as versatile building blocks for the synthesis of diverse sulphur-containing organic compounds, including sulfones, sulfonamides, and sulfonate esters. Our use of flow technology offers a robust, safe and scalable platform for effectively activating these challenging gaseous alkanes, facilitating their transformation into valuable sulfinates.
Paper details
Dmitrii Nagornîi, Fabian Raymenants, Nikolaos Kaplaneris & Timothy Noël: C(sp3)–H sulfinylation of light hydrocarbons with sulfur dioxide via hydrogen atom transfer photocatalysis in flow. Nat. Commun. 15, 5246 (2024). DOI: 10.1038/s41467-024-49322-w
See also
Website Flow Chemistry Research group