New insights into amyloid polymorphism
27 June 2024
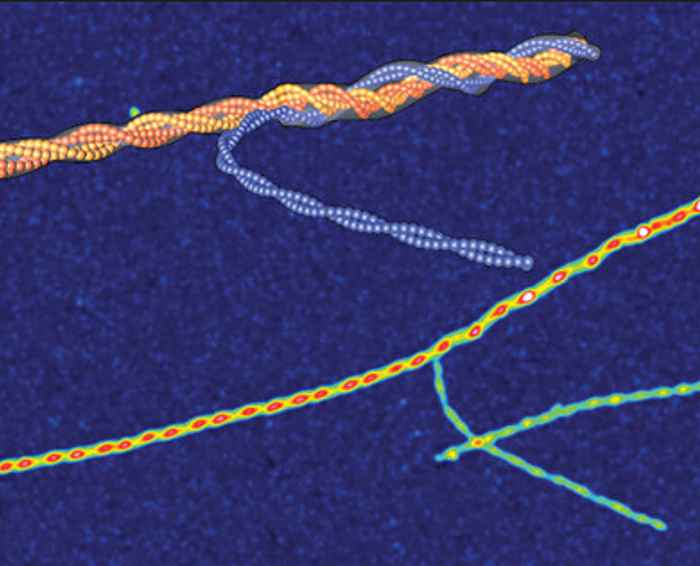
Amyloids are aggregates of proteins and polypeptides in the form of long fibres with a diameter in the nanometre range. They display a high level of polymorphism, meaning that identical polypeptides can fold into multiple distinct amyloid conformations. This rich morphological diversity across distinct amyloid species is thought to be relevant to diseases such as Alzheimer's and Parkinson's.
The underlying aggregation mechanisms and complex amyloid architectures have until now remained rather elusive. Using AFM statistical analysis combined with theoretical substantiation, the international research team has now been able to shed light on this crucial aspect of amyloid formation. The team included researchers from ETH Zurich, Ecole Polytechnique Fédérale de Lausanne (EPFL), Autonomous University of Madrid (UAM), University College London, University of Cambridge, University of Zurich, University of Wageningen and University of Amsterdam.
The researchers focused on insulin amyloid fibrils, demonstrating a rich heterogeneity of configurations, including common twisted ribbons and helical fibrils, as well as mixed-curvature polymorphs. The study reveals that amyloid polymorphism is a hallmark of almost all amyloid species and sheds light on the mesoscopic mechanisms of amyloid formation. It advances the understanding of the role of inter-protofilament interaction in the formation of both functional and pathological amyloid fibrils. In particular, the study revealed a novel fibrillization pathway involving the intertwining of protofilaments and protofibrils.
Abstract (as published in the paper)
Amyloid polymorphism is a hallmark of almost all amyloid species, yet the mechanisms underlying the formation of amyloid polymorphs and their complex architectures remain elusive. Commonly, two main mesoscopic topologies are found in amyloid polymorphs characterized by non-zero Gaussian and mean curvatures: twisted ribbons and helical fibrils, respectively. Here, a rich heterogeneity of configurations is demonstrated on insulin amyloid fibrils, where protofilament packing can occur, besides the common polymorphs, also in a combined mode forming mixed-curvature polymorphs. Through AFM statistical analysis, an extended array of heterogeneous architectures that are rationalized by mesoscopic theoretical arguments are identified. Notably, an unusual fibrillization pathway is also unravelled toward mixed-curvature polymorphs via the widespread recruitment and intertwining of protofilaments and protofibrils. The results present an original view of amyloid polymorphism and advance the fundamental understanding of the fibrillization mechanism from single protofilaments into mature amyloid fibrils.
Paper details
J Zhou, S. Assenza, M. Tatli, J. Tian, I.M. Ilie, F.S. Ruggeri, E.L. Starostin, A. Caflisch, T.P.J. Knowles, G. Dietler, H. Stahlberg, S.K. Sekatskii and R Mezzenga: Hierarchical Protofilament Intertwining rules the Formation of Mixed-Curvature Amyloid Polymorphs. Adv. Sci., 2402740 (2024). DOI: 10.1002/advs.202402740. The paper is part of Advanced Science’s Hot Topic: Amyloids
See also
- Ioana Ilie research group: Multiscale Simulations of Biomolecular Systems
- Research group Computational Chemistry