Sustainable synthesis of hydrogen peroxide
Using a cobalt-based, MOF-confined catalyst
29 July 2024
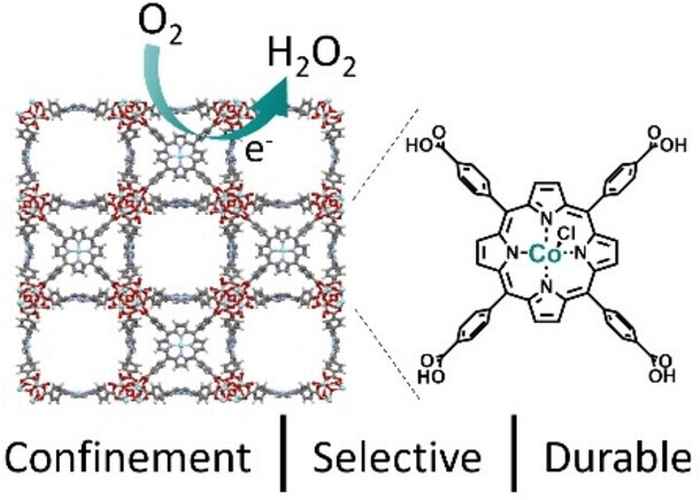
The research was performed by PhD student Dana Rademaker of Leiden University in the framework of a joint research grant obtained from the Materials for Sustainability (Mat4Sus) programme funded by the Dutch Research Council NWO. She was able to demonstrate a stable conversion for at least five hours, illustrating the potential of catalysts confined in MOFs to increase the selectivity and stability of electrocatalytic conversions.
An important bulk chemical
Hydrogen peroxide (H2O2) is an important bulk chemical that is used in various industrial processes, including paper bleaching, waste-water treatment, and the chemical synthesis of many specialty chemicals. At the same time, it is one of the most energy-intensive chemicals in the chemical manufacturing industry. Currently, it is obtained industrially using an energy- and waste-intensive anthraquinone cycling process which consumes energy of about 8 kWh/kg. Hence, sustainable alternatives for the energy intensive synthesis of H2O2 are necessary.
Electrochemical H2O2 production via the oxygen reduction reaction at neutral aqueous conditions may provide such an environmentally friendly alternative to the anthraquinone process. However, the oxygen reduction reaction towards H2O2 is in competition with the oxygen reduction reaction towards H2O. Therefore, a selective catalyst is required that promotes the selective reduction of oxygen to H2O2.
Abstract (as published in the paper)
Sustainable alternatives for the energy intensive synthesis of H2O2 are necessary. Molecular cobalt catalysts show potential but are typically restricted by undesired bimolecular pathways leading to the breakdown of both H2O2 and the catalyst. The confinement of cobalt porphyrins in the PCN-224 metal-organic framework leads to an enhanced selectivity towards H2O2 and stability of the catalyst. Consequently, oxygen can now be selectively reduced to hydrogen peroxide with a stable conversion for at least 5 h, illustrating the potential of catalysts confined in MOFs to increase the selectivity and stability of electrocatalytic conversions.
Paper details
Dana Rademaker, Stefania Tanase, Hongrui Kang, Jan Hofmann, Dennis Hetterscheid: Selective Electrochemical Oxygen Reduction to Hydrogen Peroxide by Confinement of Cobalt Porphyrins in a Metal-Organic Framework Chem. Eur. J., 2024, e202401339. DOI: 10.1002/chem.202401339
See also
- Research group Stefania Grecea: Functional Materials (Van ‘t Hoff Institute for Molecular Sciences, UvA)
- Research group Dennis Hetterscheid: Molecular Complexes for Electrocatalysis (Leiden Institute of Chemistry, Leiden University)