A sophisticated approach to watching paint dry
Striking insights obtained with time-resolved ATR-FTIR spectroscopy
5 September 2024
In both cases it is relevant to know how factors like paint composition and curing conditions affect the chemistry and network structure of the oil polymer network, and thus the long-term stability of the paint. Studying the curing process of drying oils at a molecular level, however, is no mean feat. In their study, the researchers developed an approach for using time-resolved ATR-FTIR spectroscopy on paint samples. Pushing the limits of this powerful tool, they were able to investigate the curing kinetics and evolving chemical structure, allowing quantitative monitoring of key functional groups during the entire curing process with high time resolution under a wide variety of conditions.
Surprising insights
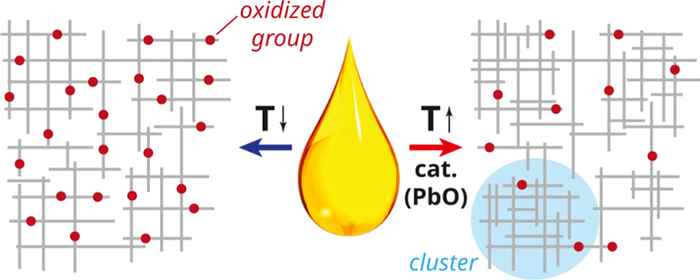
This led to a thorough understanding of many details of the curing process, and also provided a few surprising insights. For instance, the idea that the addition of a catalyst will lead to faster and more extensive curing was proven wrong in the case of a paint containing a PbO catalyst. As it turns out, high catalyst levels can result in a polymer network with less extensive global cross-linking. Another insight is that the common research practice of accelerating oil paint drying by adding catalysts and raising the temperature needs to be carefully considered, as it may induce a paint structure that is dissimilar to that of historical oil paints.
The research was partly carried out by Dr Gwen DePolo as part of a joint-degree PhD project between Northwestern University (USA) and the University of Amsterdam (UvA). She defended her thesis “Chemical and Mechanical Properties of Drying Oils during Polymerization” on 2 April, 2024. The part of the project leading to the current publication was led by Dr Joen Hermans, who holds a position as assistant professor between the Conservation & Restoration programme of the UvA Faculty of Humanities and the Chemistry for Conservation and Art research at the Van ‘t Hoff Institute for Molecular Sciences (HIMS, UvA Faculty of Science).
Abstract, as published with the paper
Drying oils like linseed oil are composed of multifunctional triglyceride molecules that can cure through three-dimensional free-radical polymerization into complex polymer networks. In the context of oil paint conservation, it is important to understand how factors like paint composition and curing conditions affect the chemistry and network structure of the oil polymer network and subsequently the links between the structure and long-term paint stability. Here, we employed time-resolved ATR-FTIR spectroscopy and comprehensive data analysis to study the curing behavior of five types of drying oil and the effects of curing temperature as well as the presence of a curing catalyst (PbO). Extracted concentration curves of key reactive functional groups point to a phase transition similar to a gel point that is especially pronounced in the presence of PbO, after which curing reactivity slows down dramatically. Analysis of kinetic parameters suggests that PbO induces a network structure with a more heterogeneous cross-link density, and the ATR-FTIR spectra indicate lower levels of oxidation in those cases. Finally, lower temperatures appear to favor the formation of carboxylic acid groups in oil mixtures with PbO.
Paper details
Gwen DePolo, Piet Iedema, Kenneth Shull, Joen Hermans: Comprehensive Characterization of Drying Oil Oxidation and Polymerization Using Time-Resolved Infrared Spectroscopy. Macromolecules, Article ASAP, Published 27 August 2024. DOI: 10.1021/acs.macromol.4c01164
See also
- Chemistry for conservation and art at the Van ‘t Hoff Institute for Molecular Sciences
- Conservation and Restoration programme at the University of Amsterdam
- Joen Hermans appointed as assistant professor of Conservation Science
- Joen Hermans’ research website