How the chain length of tetraalkylammonium cations affects the electrochemical reduction of CO2
28 August 2024
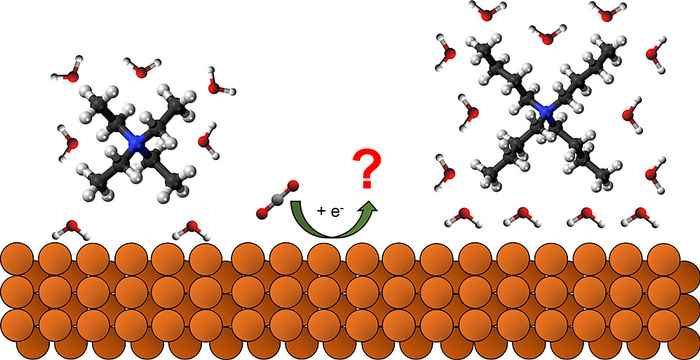
Tetraalkylammonium cations (TAA+) are widely used as supporting electrolytes in organic media due to their high solubility and conductivity. Through systematic exploration of their use in the CO2 reduction reaction (CO2RR) on copper electrodes in aprotic solvents, the HIMS electrochemistry group has now revealed how tuning their composition can enhance the efficiency and steer the conversion outcome.
The research thus paves the way for more targeted and efficient electrochemical systems, potentially offering new avenues for sustainable energy conversion and carbon capture technologies. It also highlights the critical role of cation size in modulating reaction pathways, contributing to a deeper understanding of electrochemical processes in non-aqueous media and offering promising strategies for the development of advanced CO2 reduction systems.
The research team for electrochemistry and electrocatalytic synthesis led by Dr Amanda Garcia forms part of the Heterogeneous Catalysis and Sustainable Chemistry group at the University of Amsterdam’s Van ‘t Hoff Institute for Molecular Sciences. The research is carried out both in the context of the UvA’s Research Priority Area Sustainable Chemistry and the Amsterdam Centre for Electrochemistry (Amcel).
Abstract, as published with the paper
Aprotic organic solvents such as acetonitrile offer a potential solution to promote electrochemical CO2 reduction over the competing hydrogen evolution reaction. Tetraalkylammonium cations (TAA+) are widely used as supporting electrolytes in organic media due to their high solubility and conductivity. The alkyl chain length of TAA+ cations is known to influence electron transfer processes in electrochemical systems by the adsorption of TAA+, causing modifications of the double layer. In this work, we elucidate the influence of the cation chain length on the mechanism and selectivity of the CO2RR reaction under controlled dry and wet acetonitrile conditions on copper cathodes. We find that the hydrophobic hydration character of the cation, which can be tuned by the chain length, has an effect on product distribution, altering the reaction pathway. Under dry conditions, smaller cations (TEA+) preferentially promote oxalate production via dimerization of the CO2·– intermediate, whereas formate is favored in the presence of water via protonation reaction. Larger cations (TBA+ > TPA+ > TEA+) favor the generation of CO regardless of water content. In situ FTIR analysis showed that TBA+ cations are able to stabilize adsorbed CO more effectively than TEA+, explaining why larger cations generate a higher proportion of CO. Our findings also suggest that higher cation concentrations suppress hydrogen evolution, particularly with larger cations, highlighting the role of cation chain length size and hydrophobic hydration shell.
Paper details
Connor Deacon-Price, Louis Changeur, Cássia S. Santana, and Amanda C. Garcia: The Effect of the Tetraalkylammonium Cation in the Electrochemical CO2 Reduction Reaction on Copper Electrode. ACS Catalysis, published online 14 August 2024. DOI: 10.1021/acscatal.4c02297