Unravelling the chemistry of life’s origins
2 October 2024

In the next five years, three experiments will run in front of NEMO Science Museum visitors. These will simulate a ‘primordial soup’ of simple chemicals such as ammonia, methane, water and hydrogen to create more complex chemicals, such as amino acids, that are essential to life. They are inspired by experiments conducted by scientists Stanley Miller and Harold Urey in the 1950s, which indeed yielded a range of amino acids. However, those ‘original’ experiments did not exactly match the atmosphere of the early Earth. And it was not taken into account that meteorites can deliver complex organic material from space to a planet.
Testing the meteorite hypothesis
Petrignani and NEMO now hope to obtain more meaningful results with three experiments that not only focus on the early Earth, but also on other locations where life may have developed. She is particularly excited to test the ‘meteorite hypothesis’. The first of the experiments therefore will contain amino acids and RNA molecules found on meteorites, that might have ‘spiked’ Earth’s atmosphere to develop life. ‘Whether meteorites played a role in the emergence of life, we don't know’, she explains in an accompanying article at the Nemo-Kennislink website. ‘We do know that tons of material were brought to Earth. In that sense, it is a little strange to think that it played no role at all.’

The 3-litre chemical flask in which this experiment will be performed also contains a piece of a real meteorite from the NEMO collection, but that will be mostly ‘fun for the visitors’. More relevant will be the addition of a piece of clay, testing the hypothesis that this might have provided a chemical micro-environment boosting the chemistry of the primordial soup. “The best thing would be if over time we can see clustering or polymerization of molecules,” says Petrignani. Finally, as in the original Miller-Urey experiment, lightning will be simulated by electrical sparks emanating from two electrodes.
Hot and cold
Later this year a second round-bottom flask will be installed simulating a greenhouse planet with high temperatures (oscillating between 40 and 80 degrees Celsius) and a high CO2 concentration. That might sound like hostile conditions for life, but the Earth also used to be warmer with more CO2. ‘The emergence of life probably requires more extreme conditions than its survival’, says Petrignani.
Finally, a third flask will simulate icy conditions that can be found elsewhere in our solar system, and beyond. Wherever ice is found, liquid water may have also been present. Petrignani and colleagues will therefore study the effect of freezing and melting of water on how building blocks for life may form. Currently a double-walled flask is being tested in the lab at temperatures with the minimal temperature ranging from -5 to -40 degrees Celsius.
When it will be employed at NEMO in the near future, the hope is that it can help understand the chemistry at places like the Saturn moons Titan and Enceladus, where complex organic compounds have been detected with space probes and telescopes.
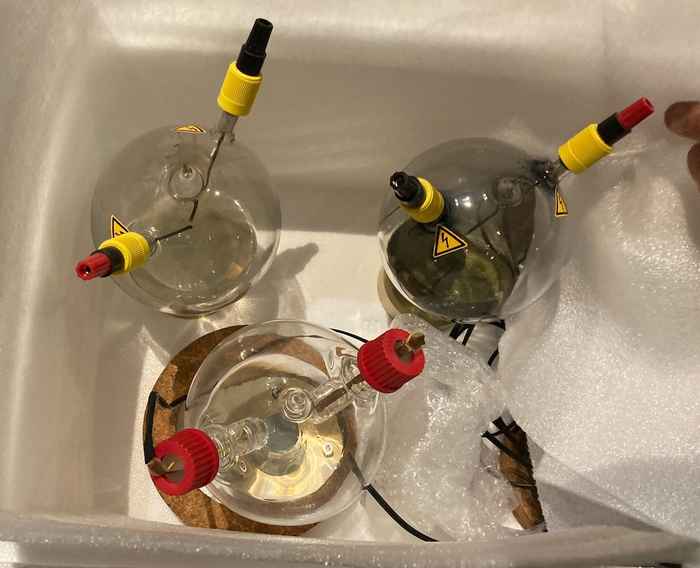
Longest running experiments
While starting the new experiments, Petrignani will also focus on the analysis of three other flasks that have been at display at NEMO in the period 2017-2024. These also contained Miller-Urey type simulations and constitute the longest-running experiments on the chemical origins of life on early-Earth. Petrignani is quite curious and excited about the building blocks of life that might be discovered after so many years. The analysis will be conducted in her POOL lab (Prebiotic Origins Of Life) at the Van 't Hoff Institute for Molecular Sciences (HIMS) by chemistry Master’s students, with support from Dr Wim Roeterdink and tapping into the expertise of HIMS colleagues in molecular photonics, analytical chemistry, organic chemistry and computational chemistry. Petrignani will also collaborate with Prof. Willem Kegel at Utrecht University.
See also
- Petrignani lab: Astro Chemistry
- Leven brouwen in bollen (NEMO Kennislink, in Dutch)
- Oersoep-experiment onverwacht succesvol (NEMO Kennislink, in Dutch)