How amines and electrodes affect CO2 conversion
Studying electrochemical CO2 reduction in an integrated system for carbon capture and utilisation
5 November 2024
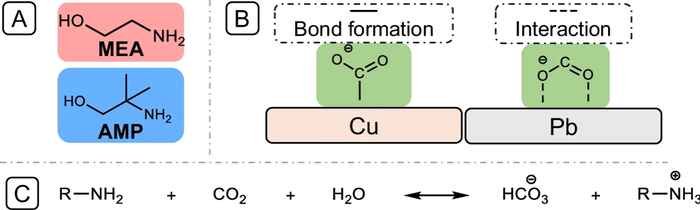
The use of amine-based solvents is key in many integrated systems for capture and subsequent electrochemical conversion of CO₂. Using in situ spectroscopy, a powerful tool for revealing reaction mechanisms, the researchers were able to pinpoint substantial differences in the reaction mechanisms between two types of amines on different electrode materials. The study revealed how the use of monoethanolamine (MEA) on copper electrodes leads to proton shuttling, which hinders CO₂ reduction. On the other hand, the researcher show how the use of 2-amino-2-methyl-1-propanol (AMP) on lead electrodes can substantially promote CO₂ reduction.
The research thus demonstrates how to optimize the choice of amines and electrode materials to promote the selective conversion of CO₂. These results are relevant to industrial application, advancing the conversion of CO₂ emissions into valuable products.
Abstract, as published with the paper
Carbon capture and utilization (CCU) technologies present a promising solution for converting CO2 emissions into valuable products. Here we show how amines, such as monoethanolamine (MEA) and 2-amino-2-methyl-1-propanol (AMP), influence the electrochemical CO2 reduction process in an integrated CCU system. Using in situ spectroscopic techniques, we identify the key roles of carbamate bond strength, proton shuttling, and amine structure in dictating reaction pathways on copper (Cu) and lead (Pb) electrodes. Our findings demonstrate that on Cu electrodes, surface blockage by ammonium species impedes CO₂ reduction, whereas on Pb electrodes, proton shuttling enhances the production of hydrocarbon products. This study provides additional insights into optimizing CCU systems by tailoring the choice of amines and electrode materials, advancing the selective conversion of CO₂ into valuable chemicals.
Paper details
Bruggeman, D.F., Rothenberg, G. & Garcia, A.C. Investigating proton shuttling and electrochemical mechanisms of amines in integrated CO2 capture and utilization. Nat Commun 15, 9207 (2024). DOI: 10.1038/s41467-024-53543-4