NWO XS Grant for biosensor development
Pioneering a new approach to whole blood diagnostics
5 November 2024
The grant, awarded by the Dutch Research Council NWO, will support groundbreaking research aimed at detecting disease biomarkers in whole blood samples. Such tests hold the promise of simplicity and speed and reducing costs. However, their reliability is often compromised by the optical inhomogeneities present in the samples. Mutti and coworkers propose a novel sensing strategy to overcome this hurdle.
Engineered enzymes

The research builds upon the expertise of the HIMS Biocatalysis group in the field of engineered enzymes. These can be used as crucial recognition units of biosensors, improving sensitivity and selectivity. Immobilized on suitable materials, the enzymes can be used for the highly reliable detection of biomarkers even when these molecules are present at very low concentrations within complex biological mixtures. Moreover, enzymes can increase robustness, allowing for long-term storage at ambient temperature and recyclability.
Such innovative biosensors thus hold the promise of cost-effective screening procedures without the need for expensive equipment or specialized medical staff. They facilitate monitoring the concentration of disease biomarkers over time, allowing for the early diagnosis of potential diseases. This is relevant to developed countries, with their ageing population and increasing pressure on the healthcare systems, as well as developing countries which often lack access to sophisticated diagnostic equipment while facing a higher burden of infectious diseases. Enzyme-based biosensors can be simple, affordable, and easy to use – even in resource-limited remote areas with minimal infrastructure.
Precise measurement
At the Biocatalysis group, the NWO XS grant will support the development of an innovative approach to sensing by means of fluorescence ratiometry. Although this is an established sensing method, its performance declines in the case of whole blood samples. There, the optical inhomogeneity of the sample impedes the comparison of response and reference signals of different wavelengths, thus rendering results unreliable.
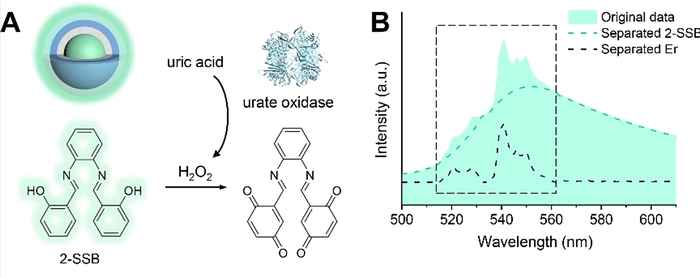
Dubbed machine-learning-assisted fluorescence ratiometry, the novel approach promises superior diagnostic accuracy by simultaneously measuring both response and reference emission signals at the same wavelength. The primary challenge here is separating the response from the reference emissions, which will be tackled using machine learning linear regression analysis based on the emission peak shape. This will enable the precise measurement of the ratio between response and reference emissions, offering quantitative results for whole blood samples and in vivo applications.
The proposed biosensor will be composed of nanophosphor materials with an organic fluorophore chemically linked as the probe. Importantly, the organic fluorophore can exist in two redox forms: a fluorescence-responsive reduced form and a non-responsive oxidized form. By functionalizing the nanoprobe with any H2O2-producing enzyme (e.g., from the oxidase family), it will be possible to detect a wide range of disease biomarkers, highlighting the universality of the envisioned biosensing platform.
See also
- HIMS research group Biocatalysis
- NWO news item: 28 grants within Open Competition ENW-XS