Transient modulation of catalysis by switching effectors in molecular cages
Novel catalysis concept provides outlook on paradigm shift in chemical conversion
4 February 2025
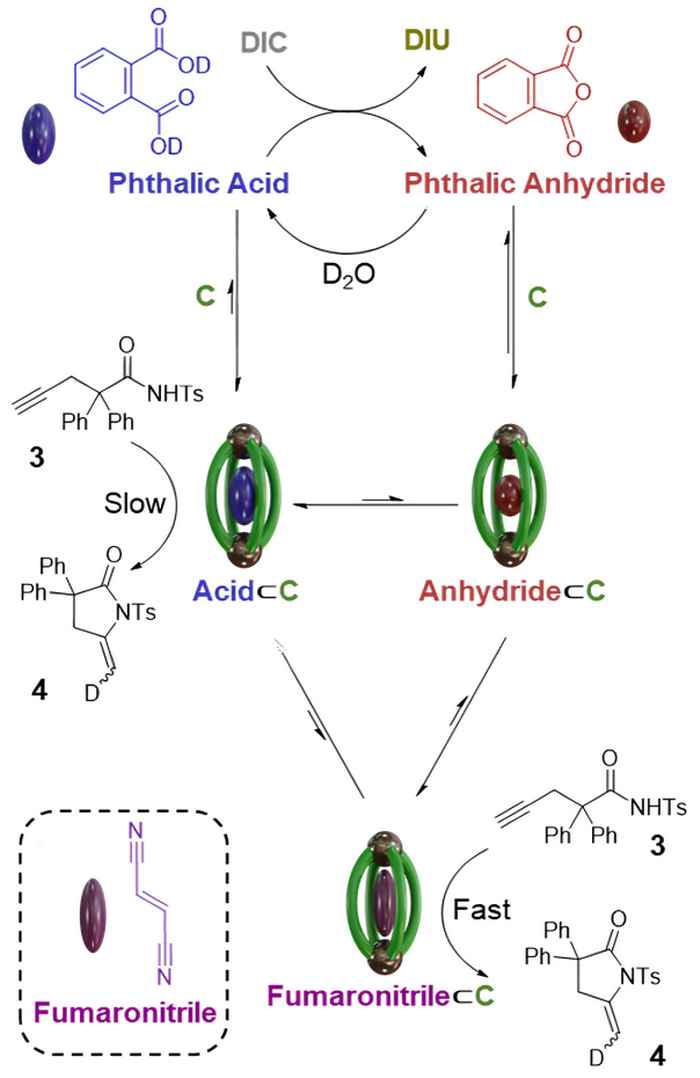
The authors used the Pt2L4 molecular cage which catalyzes a cyclization reaction at the outside of the cage. The guest molecules that bind inside the cage alter the reaction rate that takes place outside the cage by changing the electronic properties of the metal.
Guest molecules can be generated by an independent diacid-catalyzed carbodiimide hydration cycle. The researchers demonstrate how the latter, orthogonal reaction influences the rate of the Pt-catalyzed cyclization reaction to different extents. The guest molecules, that act as effectors to the cyclization reaction, are transiently generated by the carbodiimide driven cycle and thus regulate transiently the turnover frequency of the catalyst.
This novel concept opens up a systems chemistry approach where multi-component supramolecular architectures can be used to modulate chemical conversions, for instance by biasing different reaction pathways, or introducing cooperative effects to amplify small changes.
More efficient and self-regulating chemistry
The novel concept can help introduce a paradigm shift in chemical production, which is currently based on stepwise synthesis routes using pure components in a controlled, isolated environment. In contrast, biochemical conversions in nature take place in very complex mixtures facilitating all relevant conversion steps. This involves supramolecular control over reaction networks and molecular feedback loops. Emulating such biochemical systems can not only enhance overall efficiencies but also introduce autocorrection features. The latter is very important, for example, in the sustainability transition where oil is replaced with bio- and waste-based feedstocks that often fluctuate in composition and quality. The envisioned self-regulating conversion systems will result in a much more stable conversion of such feedstocks.
Abstract, as published with the paper
The complexity of allosteric enzymatic regulation continues to inspire synthetic chemists seeking to emulate interconnected biological systems. In this work, a Pt2L4 cage capable of catalyzing the cyclization reaction of an alkynoic tosyl amide is orthogonally coupled to a diacid-catalyzed carbodiimide-hydration cycle. This new Pt-catalyzed cyclization reaction is demonstrated to exhibit electronic regulation by inclusion of different guest effectors. The orthogonal diacid-catalyzed carbodiimide hydration cycle produces transiently diverse guests that influence the rate of the Pt-catalyzed cyclization reaction to different extents. Further complexity can be introduced to the system through displacing the transiently-formed, weakly bound anhydride guest with the stronger binding fumaronitrile, affecting the catalytic rate to a larger extent for the duration of the orthogonal reaction cycle. The modulation of a Pt-catalyzed cyclization reaction can thus be regulated transiently over the course of the reaction—either up- or down-regulating the turnover frequency (TOF)—via coupling with a temporally controllable orthogonal process. This study demonstrates that principles of allosteric enzymatic regulation can also be applied to simple artificial systems.
Paper details
Zoe Ashbridge, Joost N. H. Reek: Transient Allosteric Regulation of Catalysis by Effector Switching in a Pt2L4 Cage. Angew. Chem. Int. Ed. 2025, e202500214 DOI: 10.1002/anie.202500214
See also
Research group Homogeneous, Supramolecular and Bio-inspired Catalysis