Direct late-stage alkylation of heteroarenes using gaseous alkanes
3 June 2025
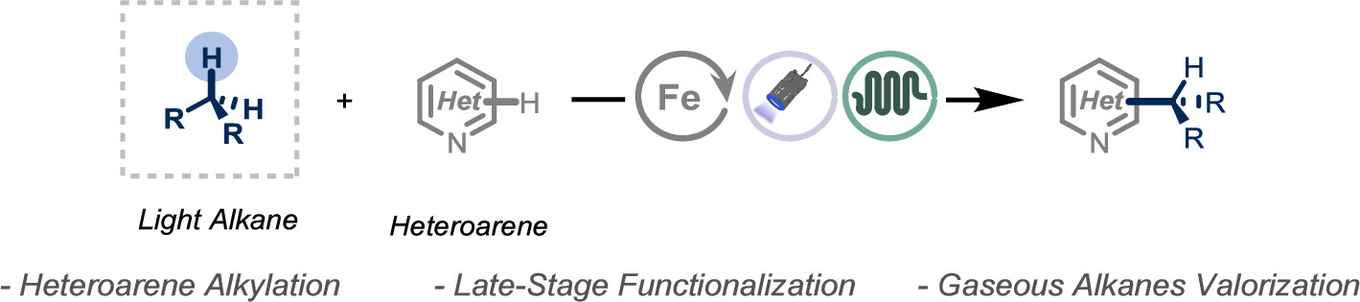
Late-stage functionalization enables the rapid diversification of complex molecules. However, until now the direct use of gaseous C1–C4 hydrocarbons for such functionalization has been largely out of reach. In their ACS Chemical Science paper, the Flow Chemistry researchers describe how they change this using a photocatalytic, continuous-flow system for performing a Minisci-type reaction that directly alkylates heteroarenes using simple gases like methane, ethane, propane, and butane. Based on hydrogen atom transfer (HAT), it does not need pre-functionalized reagents so that the simple switching of the gas supply results in a change of alkyl group added in the functionalization. The concept was demonstrated by synthesizing six alkylated analogues of the fungicide Quinoxyfen.
Abstract, as published with the paper
The late-stage functionalization of complex molecules is a pivotal strategy in drug discovery, enabling the rapid optimization of lead compounds. However, the use of gaseous alkanes as alkylating agents in these processes remains underexplored due to their inertness and handling challenges. Here we present a photocatalytic platform that facilitates the alkylation of heteroarenes using abundant gaseous C1–C4 hydrocarbons under continuous-flow conditions. Through hydrogen atom transfer (HAT) catalysis, we achieve the efficient alkylation of pharmaceutically relevant compounds without the need for prefunctionalized reagents. Our method is not only scalable and sustainable but also extends to the functionalization of marketed drugs and natural products.
Paper details
Prakash Chandra Tiwari, Antonio Pulcinella, Emil Hodžić, and Timothy Noël: Late-Stage Heteroarene Alkylation via Minisci Reaction with Gaseous Alkanes Enabled by Hydrogen Atom Transfer in Flow. ACS Central Science, Article ASAP. DOI: 10.1021/acscentsci.5c00468