Novel robust and scalable method for selective methylation of (hetero)aryl bromides
30 June 2025
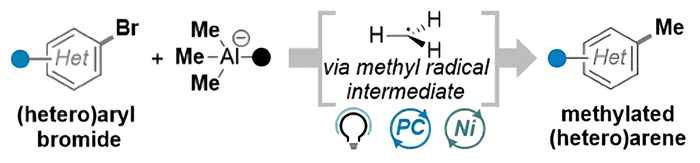
Compared to previous methods, the novel strategy leverages a commercially available, air-stable aluminium-based reagent and an organophotocatalyst to enable a simplified, fast, and robust procedure for selective C(sp²)–CH₃ bond formation, expanding the toolkit for modern cross-coupling chemistry. It accommodates a broad range of functionalities, delivering clean methylation without protodehalogenation. Furthermore, the advantages of continuous-flow technology offer opportunities for efficient scale-up with improved process metrics.
Abstract, as published with the paper
We report a metallaphotocatalytic strategy for the selective methylation of (hetero)aryl bromides via nickel-catalyzed cross-coupling with bis(trimethylaluminum)-1,4-diazabicyclo[2.2.2]octane (DABAl-Me₃), as a commercially available, air-stable, and non-pyrophoric aluminum-based reagent. The method enables a fast, robust, and scalable methylation protocol that broadly accommodates various functional groups while preventing protodehalogenation. Mechanistic studies confirm the unprecedented generation of methyl radicals from an organo-aluminum precursor under photoredox conditions, bypassing the limitations of conventional two-electron pathways. This work expands the toolbox of practical radical precursors and provides a streamlined approach for selective C(sp2)─CH3 bond formation.
Paper details:
Djossou, J., Aloia, A., Capaldo, L., Snabilié, D. D., Regnier, M., Schuurmans, H. A., Monopoli, A., & Noël, T.: Rapid Methylation of Aryl Bromides Using Air-Stable DABCO- Bis(Trimethylaluminum) via Nickel Metallaphotoredox Catalysis. Angewandte Chemie International Edition, e202508710. DOI: 10.1002/anie.202508710
See also