Advanced catalysts for light-triggered curing of dark alkyd paints
26 August 2025
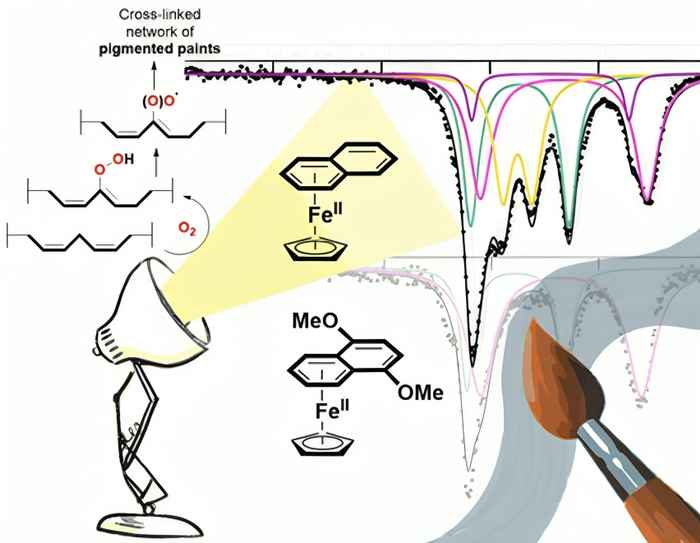
The study advances the field of light-activated catalysis for alkyd paint applications. While traditional (Cp)Fe(benzene) complexes work effectively in clear formulations, they struggle with dark-pigmented paints. The paper shows that using naphthalene and methoxynaphthalene ligands significantly enhances light absorption and catalytic efficiency. This improvement translates into better latency control and makes it possible to activate catalysts even in the deeper regions of pigmented coatings, thereby widening applicability in real-world coatings.
he PhD research of Jens Tolboom is supervised by Prof. Bas de Bruin at the research group Homogeneous, Supramolecular and Bio-inspired Catalysis (HomKat). The project was carried out in collaboration with HomKat postdoctoral researcher Johan Bootsma, Akzo Nobel Decorative Coatings (Jitte Flapper) and the Inorganic Chemistry Laboratory at the University of Oxford (Alexis K. Bauer & Prof. Michael L. Neidig). It is part of the Advanced Research Center for Chemical Building Blocks, ARC CBBC.
Abstract, as published with the paper
The class of photoactive complexes [(Cp)FeII(arene)]+ (Cp = cyclopentadienyl, arene = C6H6, C6H5;Me) enable light-controlled alkyd paint curing, eliminating the need for anti-skinning agents. While [(Cp)FeII(benzene)](PF6) (0.10 wt%) initiates curing under ambient light (116 Lux/1.7 mW/m2) within 6 h for transparent paints, it is ineffective in dark-colored formulations. Here, we demonstrate that the more photosensitive complexes [(Cp)FeII(L)](PF6) (L = naphthalene, 5-methoxynaphthalene, 6-methoxy-naphthalene, 5,8-dimethoxynaphthalene) accelerate curing with lower loadings (0.05 wt%). Due to their higher extinction coefficients and red-shifted adsorption bands, these [(Cp)FeII(L)](PF6) catalysts effectively cure dark-pigmented paints. Mössbauer spectroscopy reveals the formation of a high-spin FeII intermediate as the active species. TD-DFT calculations reveal a redshift in absorption, attributed to a transition from dz[2]-dxy*/dyz* (arene/naphthalene) to Lπ-dxy*/dyz* (methoxylated naphthalenes). Excitation of the complexes using wavelengths corresponding to these absorbance bands result in weakening of the FeII-η6C6 bonds, facilitating light-induced dissociation. This advancement enhances latency originating from photoactivation while expanding applicability to pigmented systems.
Paper details
Jens Tolboom, Alexis K. Bauer, Johan Bootsma, Jitte Flapper, Michael L. Neidig, Bas de Bruin: Advancing Light-Triggered Alkyd Paint Curing: (Cp)FeII(naphthalene)](PF6) Catalysts with Enhanced Photosensitivity and Increased Performance in Pigmented Alkyd Paints Chemistry Europe, 2025, e202500104 First published: 06 July 2025 DOI: 10.1002/ceur.202500104