Can nanoplastics change protein structure and aggregation?
NWO-M funding for Sander Woutersen
19 August 2025
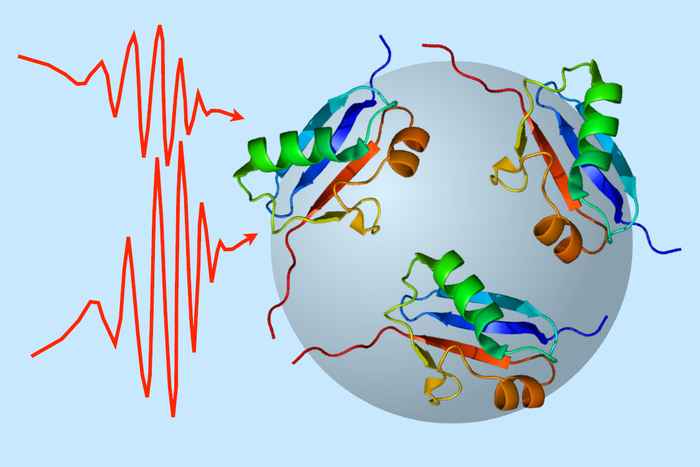
There is increasing evidence on the biological effects of nanoplastics, affecting our health in multiple ways. However, comparatively little is known about the interactions between nanoplastics and the all-underlying molecular machinery of our body: proteins. Sander Woutersen wants to change that. In a new project that was recently awarded funding from the ‘Open Competition Domain Science-M’ scheme of the Dutch Research Council NWO, Woutersen proposes to use state-of-the-art infrared spectroscopy to investigate how plastic nanoparticles interact with proteins and change their conformation - their spatial structure.
In particular, he wants to establish whether and how this interaction might have an effect on the formation of amyloids. These are fibrous deposits within and around cells that form from proteins that have lost their native conformation. Such protein misfolding, aggregation and deposition can disrupt the healthy function of tissues and organs. For instance with Parkinson's disease, there are studies suggesting that nanoplastics may influence the amyloid aggregation that underlies the pathogenic process.
Diffusion ordered spectroscopy
The NWO funding will enable Woutersen to appoint a PhD candidate who will focus on nanoparticles of the most common plastics and study their interaction with a set of representative proteins. Among these is alpha-synuclein, a protein that can cause amyloid formation associated with Parkinson's disease. Various mixes of particles and proteins will be studied using methods for size-sensitive infrared (IR) and two-dimensional infrared (2D-IR) spectroscopy that Woutersen and his co-worker Giulia Giubertoni have recently developed.
A crucial aspect of the study is the application of the principle of infrared diffusion ordered spectroscopy or IR-DOSY, which enables the separation of molecules in a sample based on their diffusion behaviour. This will enable to distinguish the infrared spectra of proteins bound to nanoplastic particles from those of unbound proteins (the latter will display a much faster diffusion).
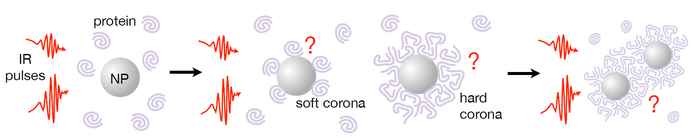
Comparing the IR and 2D-IR spectra of these different species will help understand how nanoplastics affect the conformation of a protein upon adsorption to a nanoparticle, how this changes as the adsorbed protein layer (corona) around a nanoparticle grows in thickness, and how amyloid formation is affected by the presence of nanoplastics. In particular regarding the behaviour of alpha-synuclein, Woutersen will cooperate with prof. Mireille Claessens of Nanobiophysics at the University of Twente, who studies alpha-synuclein, its function and aggregation, and its relation to conditions such as Parkinson's disease.