Novel catalyst for CO2 conversion is free of traditional transition metals
26 November 2025
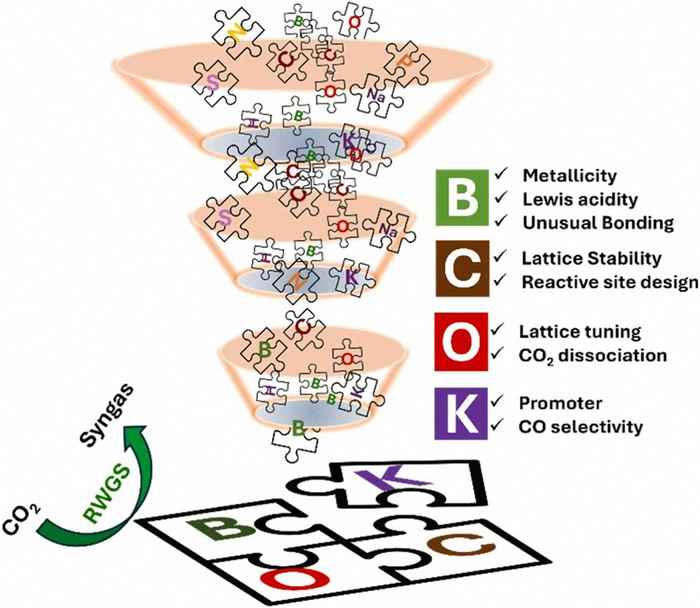
The development of the new catalyst extends the group’s prior research on MXene- and MAX-phase materials. Applying the design principles of layered nanosheets, the researchers created a metal-free, boron-based catalyst system that delivers stable and selective performance.
First author of the paper is Dr Anju Rajamohanan Sobhana, who started her research in early 2024 as a Marie Curie postdoctoral fellow, leading the project ‘2D BoroCat’.
Towards more sustainable, circular chemistry
The Reverse Water Gas Shift reaction offers a pathway for converting CO2 into valuable chemical building blocks. In this reaction, CO2 is reduced to carbon monoxide (CO), producing syngas, a mixture of CO and H2. Currently produced mainly from fossil-based feedstocks, syngas plays a pivotal role in many existing chemical processes. Producing syngas from CO2 can thus enable a transition of traditionally fossil-based chemical processes towards more sustainable and circular pathways.
Crucial to the RWGS reaction are catalysts facilitating the CO2 conversion. These can be based on noble metals that offer excellent performance but feature high cost and limited availability. Catalysts from alternative metals such as copper, nickel and iron are more affordable but suffer from issues such as poor selectivity and metal particle aggregation leading to catalyst deactivation.
Boron nano sheets

Guided by the concept of rational material design through targeted element selection, Anju Sobhana and co-workers developed a novel class of catalysts with boron at their core. It is present in nano-sheets to which other elements are introduced to optimise catalytic performance: carbon helps boost stability and activity, oxygen enhances efficiency, and potassium improves selectivity. Together, these components create a catalyst engineered for optimum performance.
After optimising the design and preparation of the catalyst, the researchers applied the catalyst for the RWGS reaction, demonstrating a truly metal-free RWGS catalyst achieving high performance under ambient pressure. The findings demonstrate the potential of this class of catalysts as robust, scalable, and sustainable alternatives to state-of-the-art transition-metal-based catalysts for CO2 valorisation. The researchers anticipate that these findings will provide a foundation for the design and activity of metal-free catalysts applicable to a diverse range of chemical transformations.
International collaboration
This project represents a truly international collaboration, bringing together expertise from leading institutions across Europe and Asia. The diverse network combines complementary expertise in materials characterisation, catalysis and computational chemistry, fostering cross-border knowledge exchange and accelerating the development of next-generation catalysts for CO2 valorisation.
The team includes Pankaj Kumar and Vimal Chandra Srivastava from the Indian Institute of Technology Roorkee, Dhanaji R. Naikwadi and Atul Bansode from Delft University of Technology, Merel C. Konings and Freek Ariese from Vrije Universiteit Amsterdam, Bettina Baumgartner and Pascal Stam from the University of Amsterdam, Fengshou Yu from Hebei University of Technology-China, Erdni D. Batyrev from Tata Steel Research & Development in the Netherlands, and Paula Oulego from the University of Oviedo in Spain.
Paper details
Anju, R. S., Kumar, P., Naikwadi, D. R., Konings, M. C., Stam, P. G., Ariese, F., Baumgartner, B., Bansode, A., Yu, F., Batyrev, E. D., Oulego, P., Srivastava, V. C., & Shiju, N. R. (2025). Beyond Metals: Tailored Metal-Free Boron-Oxy-Carbide Catalysts for CO2 Hydrogenation. Applied Catalysis B: Environment and Energy, 384, 126153, 2026. DOI: 10.1016/j.apcatb.2025.126153
Earlier research papers
M. Ronda-Lloret, T. K. Slot, N. P. van Leest, B. de Bruin, W.G. Sloof, E. Batyrev, A. Sepúlveda-Escribano, E.V. Ramos-Fernandez, G. Rothenberg, N.R. Shiju, The Role of Vacancies in a Ti2CTx MXene-Derived Catalyst for Butane Oxidative Dehydrogenation. ChemCatChem 14 (18), 2-7, 2022. DOI: doi.org/10.1002/cctc.202200446
M. Ronda-Lloret, L. Yang, M. Hammerton, V. S. Marakatti, M. Tromp, Z. Sofer, A. Sepúlveda-Escribano, E. V. Ramos-Fernandez, J. J. Delgado, G. Rothenberg, T. R. Reina and N. R. Shiju, Molybdenum Oxide Supported on Ti3AlC2 is an Active Reverse Water-Gas Shift Catalyst, ACS Sustainable Chem. Eng., 9, 4957-4966, 2021 DOI: 10.1021/acssuschemeng.0c07881