Formate as a key intermediate in carbon dioxide utilization
18 November 2022

Replacing fossil feedstocks for chemicals and polymers in the chemical industry is a key step towards a future circular society. CO2 offers a great opportunity as a starting material for new fossil-free value chains. However, since CO2 has a very low reactivity, its conversion requires either high energy inputs or nifty catalytic systems. An example of the latter can be found in an electrochemical cell using a gas-diffusion electrode to convert CO2 into formate. To further fulfil the promises of this first step in CO2 utilization, a conversion path needs to be developed that connects formate to existing or novel chemical synthesis routes suitable for large-scale industrial application.
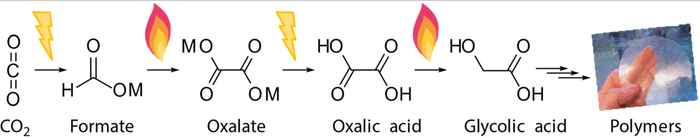
In their perspective article, Schuler and co-workers focus on the coupling of two formates to oxalate and explore alternative coupling partners for formate to unlock its full potential. Oxalate or oxalic acid is considered a potential versatile ‘platform chemical’ for materials production. It can for instance be further converted towards glycolic acid and other useful monomers that enable the synthesis of renewable plastics. Thus, the formate-to-oxalate coupling reaction, or FOCR, can be considered as a crucial step in the chemical conversion route from CO2 towards valuable products.
Most recent advances and challenges
As the paper describes, the FOCR has been studied for more than 175 years and has even seen an industrial use in the past. It presents the history of the FOCR, discusses the most recent advances and challenges for its implementation in CO2 utilization processes in the future. The authors provide an overview of the various reaction parameters and their ability to influence the reaction and products that can be obtained. To understand the reaction better and improve it in the future, they critically discuss the many mechanisms proposed for the various catalytic systems in the FOCR. Finally, they explore the potential to introduce new catalytic and solvent systems or co-reactants to the FOCR, to improve reaction performance and broaden the range of products from CO2 derived formate.
Paper details
Eric Schuler, Michele Morana, Pavel A. Ermolich, Kristian Lüschen, Adam J. Greer, S. F. Rebecca Taylor, Christopher Hardacre, N. Raveendran Shiju and Gert-Jan M. Gruter: Formate as a key intermediate in CO2 utilization Green Chem.,2022, 24, 8227-8258 DOI: 10.1039/D2GC02220F, cover: 10.1039/D2GC90097A
See also
- Research group Industrial Sustainable Chemistry
- Research group Catalysis Engineering