Research
Functional Materials group
The research includes:
- Materials design, characterisation and methodology development;
- Porous materials for molecular separation, molecular storage and catalysis;
- Molecular sensing and chemical detection;
- Proton conductive materials for fuel cell applications;
- Fundamental understanding of chemical transformations within confined spaces.
Molecular separations
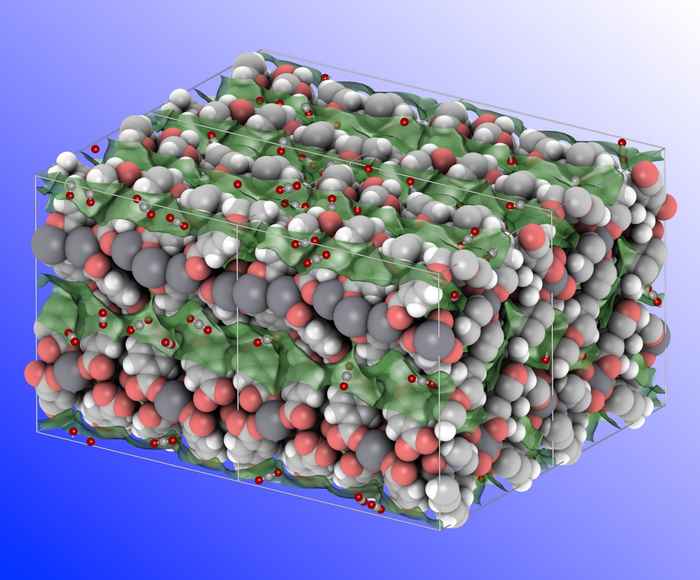
The separation and purification steps cost a staggering amount of energy worldwide. The technology (mainly distillation) is mature and low-risk, and therefore companies keep on using it. However, distillation is unpractical when the boiling points are close or when the liquid mixtures are composed of reactive components. One alternative for distillation is adsorptive separation using porous solids. The driving forces are kinetic, enthalpy and, most promising, entropic factors.
We develop synthetic strategies for making crystalline microporous materials and their corresponding composites membranes for removing the water from alcohol-water mixtures. This research is highly relevant for bio-fuels purification and air drying. We are also designing new materials for separating saturated, unsaturated and aromatic hydrocarbon mixtures. Using predictions from state-of-the-art molecular simulations (performed in the group of Dr David Dubbeldam at the University of Amsterdam), we design and synthesise porous materials with tuneable pore geometries and network topologies. These materials are tested in separating C4 and C8 hydrocarbon mixtures under simulated industrial scenarios.
Molecular sensing
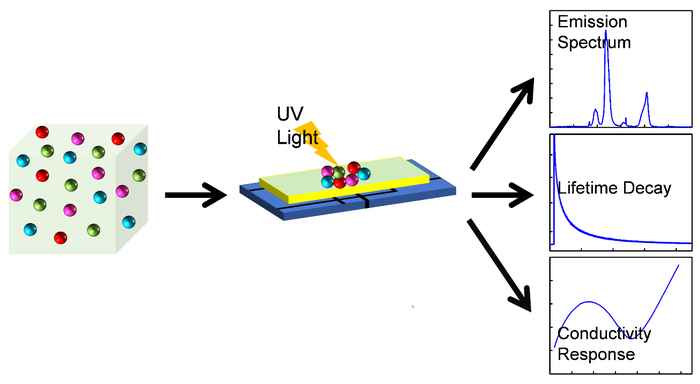
Sensor devices play a key role in industry and modern life, including process engineering and monitoring, medical technology, automotive and aerospace industry, and in safety and security technology. State-of-the-art sensing devices, using inorganic oxides or organic polymer materials, still lack advanced sensitivity and selectivity, therefore limiting key applications.
We aim at developing nanostructured lanthanide-based microporous materials which can be used as optically responsive sensors. The host-guest interactions within these materials allow the pre-concentration of analyte molecules within the pores, which is responsible for highly sensitive detection. We control the selective detection by tuning the functionality of the pore walls. This promotes preferred analyte binding, affording selective molecular detection.
We work on various projects in close collaboration with the Holst Center, Co van Ledden Hulsebosch Center (Prof. Arian van Asten) and VU (Dr Elizabeth von Hauff).
Proton conductive materials
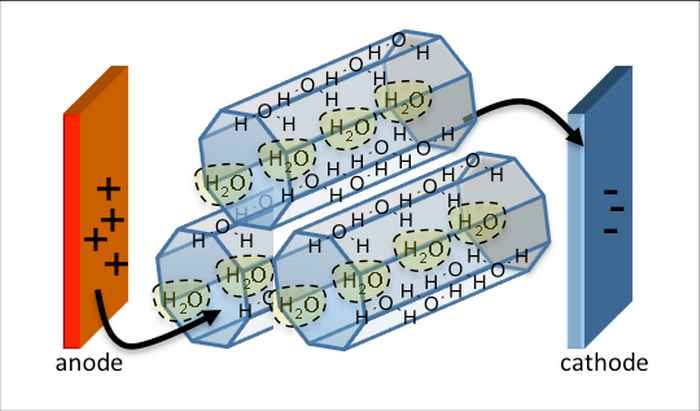
Proton exchange membranes fuel cells turn the chemical energy of fuel into electrical energy without fuel combustion. This is a promising technology for clean and efficient power generation. State-of-the-art cells use Nafion-type membranes. These, however, have two thorny limitations: First, they are prone to dehydration, losing their proton conductivity above 80 °C. Second, they are expensive, preventing large-scale commercial applications.
We design porous materials for low-temperature exchange membranes fuel cells and we carry out studies to understand the mechanisms of the proton conductivity. We further process these materials as composite membranes and study their performances in conditions relevant to real-life applications.
Catalysis in confined spaces
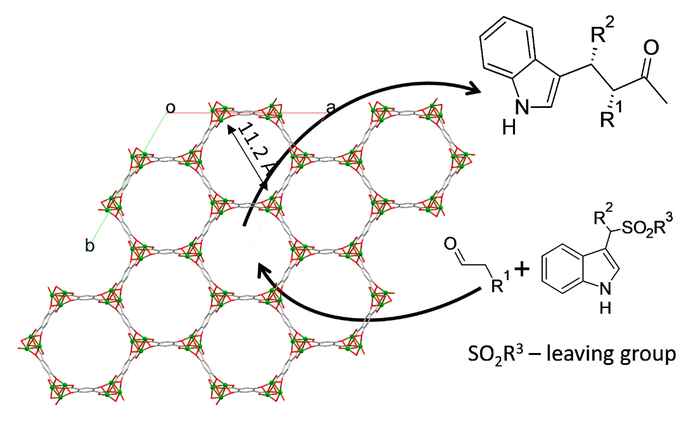
Asymmetric catalysis plays a key role in modern chemistry, especially in the field of pharmaceuticals. Homogenous catalysts are usually employed because of their excellent activities and selectivities. Using heterogeneous catalysts has the main advantage of easy catalyst separation from the product stream. This would facilitate the implementation of continuous chemical processes.
We aim at developing synthetic strategies for designing basic chiral MOF materials, which can be used as heterogeneous catalysts in aminocatalytic alkylations. Such materials offer great catalytic efficiency and economy in contrast to the bulky heterogeneous catalysts that have catalytically active sites only at the solid surface. The intrinsic porous structure of MOFs imposes a special confinement with the catalytic reaction taking place in a chemically and sterically tunable environment.